Figures & data
Figure 1. Participant disposition
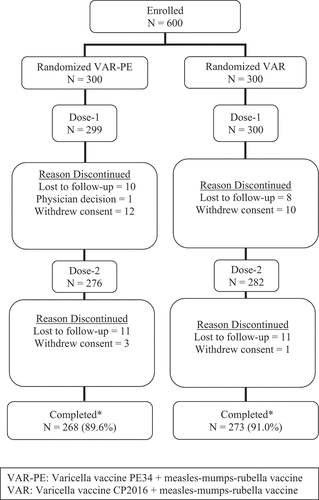
Table 1. Demographics
Table 2. Summary of VZV response rates and geometric mean titers (GMTs) at 6 weeks postdose 1
Table 3. Adverse Experience (AE) Summary (Days 1 to 42 Postdose 1 and 2)
Table 4. Summary of participants with VRC-solicited injection site varicella vaccine related adverse events
Table 5. Summary of Participants with Systemic Adverse Events (incidence ≥10% in either group)
Table 6. Analysis of rates of fever by Day Range between vaccination groups (all participants as treated population)
