Figures & data
Table 1. Subject demographics in the MenACWY-TT booster phase (booster total vaccinated cohort).
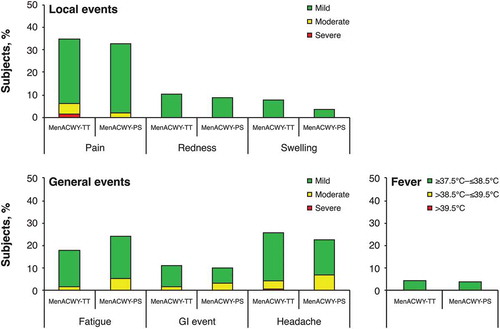
Figure 1. Percentages of subjects with booster responses for serogroups A, C, W, and Y at 1 month after booster dose of MenACWY-TT (booster ATP cohort). ATP = according-to-protocol; MenACWY-PS = quadrivalent meningococcal polysaccharide vaccine; MenACWY-TT = quadrivalent meningococcal tetanus toxoid conjugate vaccine.
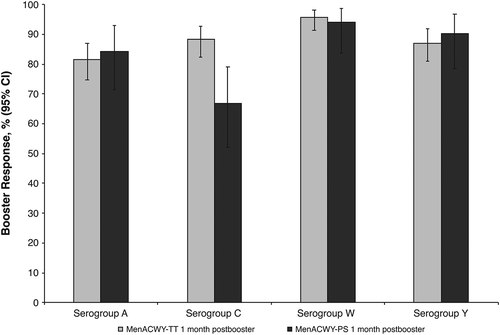
Table 2. GMTs for serogroups A, C, W, and Y before and 1 month after a booster dose of MenACWY-TT (booster ATP cohort).
Table 3. Percentage of subjects with anti-tetanus toxoid concentrations ≥0.1 IU/mL and ≥1.0 IU/mL and GMCs before and 1 month after booster dose of MenACWY-TT (booster ATP cohort).
Figure 2. Percentages of subjects with rSBA titers (A) ≥1:8 and (B) ≥1:128 at Year 10 (before booster) and at 1 month after a booster dose of MenACWY-TT (booster ATP cohort). ATP = according-to-protocol; MenACWY-PS = quadrivalent meningococcal polysaccharide vaccine; MenACWY-TT = quadrivalent meningococcal tetanus toxoid conjugate vaccine; rSBA = serum bactericidal antibody assay using baby rabbit complement.
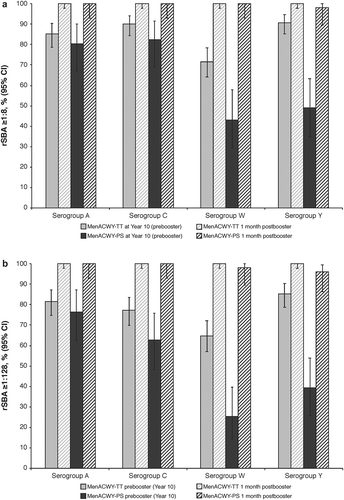
Figure 3. Percentages of subjects reporting solicited local and general events during 4-day follow-up to booster vaccination (booster total vaccinated cohort). Intensity scale (mild, moderate, or severe) was classified by grade 1, 2, or 3, respectively, for pain, fatigue, GI event, and headache; and 0–≤20 mm, >20–≤50 mm, or >50 mm for redness and swelling. GI = gastrointestinal; MenACWY-PS = quadrivalent meningococcal polysaccharide vaccine; MenACWY-TT = quadrivalent meningococcal tetanus toxoid conjugate vaccine.