Figures & data
Figure 1. The number of H. pylori in the gastric tissue of immunized and unimmunized (control) mice as detected by quantitative bacteriology. The stock of H. pylori SS1 was cultured on goat blood agar plates for 72 h in a microaerophilic environment, and then cultured for another 18 h in Brucella broth supplemented with 10% fetal calf serum with shaking at 150 rpm. The optical density of H. pylori liquid culture was adjusted to 1.0 (~1.5 x 1010 CFU/ml), and used in mouse challenge experiments. Groups of immunized and unimmunized mice were orally challenged with 3 × 109 CFU of H. pylori SS1 2 weeks after the last immunization, and the mice were sacrificed 5 weeks after challenge. Serial dilutions of the stomach homogenates were plated on Helicobacter-selective plates. After 72 h of incubation at 37°C under microaerophilic conditions, visible colonies with typical H. pylori morphology were counted. Plates with 30 to 300 colonies were used for calculating the number of bacteria per stomach by multiplying by the appropriate dilution factor. The detection limit of the assays is log10 2.2/g stomach tissue. Data are presented as mean ± SEM and are representative of one of two independent experiments with similar results
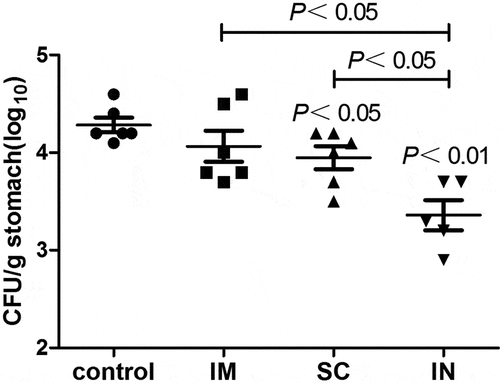
Figure 2. Serum antigen-specific IgG (A) and IgG1/IgG2a (B) responses in mice immunized with cGAMP adjuvanted H. pylori subunit vaccine by different parenteral routes. Groups of BALB/c mice (n = 6) were immunized with cGAMP adjuvanted H. pylori vaccine containing 10 µg of each UreA, UreB and NAP by the indicated route on days 0 and 14. The mice were orally challenged with 3 × 109 CFU of H. pylori SS1 at day 28, and sacrificed 5 weeks after challenge. The blood was collected and serum samples were prepared and assayed by ELISA. Serially diluted serum (10-fold) and gastric tissue homogenate supernatant (2-fold) were added to 96-well ELISA plates (100 µl/well), and incubated at 37°C for 1 h. After washing, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG, IgG1, or IgG2a (Southern Biotech, Birmingham, AL, USA) secondary antibody was added and incubated for 30 min. The wells were washed again before the addition of TMB substrate. The plates were incubated at 37°C for 15 min, the enzymatic reaction was stopped by adding 50-µl 2 M sulfuric acid, and the absorbance at 450 nm was then measured in a spectrophotometer (MultiskanMK3 plate reader, Thermo Scientific, Waltham, MA, USA). Data are presented as mean ± SEM and are representative of one of two independent experiments with similar results
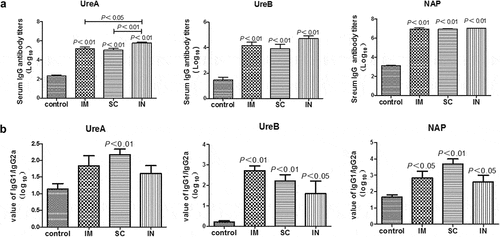
Figure 3. Antigen-specific IgA responses in the stomach (A) and serum (B) of mice immunized with cGAMP adjuvanted H. pylori subunit vaccine by different parenteral routes. Groups of BALB/c mice (n = 6) were immunized with cGAMP adjuvanted H. pylori vaccine containing 10 μg of each UreA, UreB, and NAP by the indicated route on days 0 and 14. The mice were orally challenged with 3 × 109 CFU of H. pylori SS1 at day 28, and sacrificed 5 weeks after challenge. The stomachs and blood were collected and samples were prepared and assayed by ELISA as described in detail in legend, except that HRP-conjugated goat anti-mouse IgA (Southern Biotech, Birmingham, AL, USA) was used as secondary antibody. Data are presented as mean ± SEM and are representative of one of two independent experiments with similar results
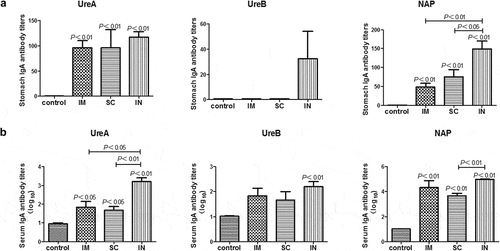
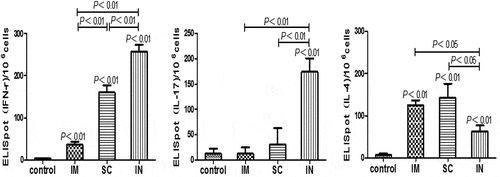
Figure 4. Antigen-specific cytokine-producing cell frequencies in mice immunized with cGAMP adjuvanted H. pylori subunit vaccine by different parenteral routes. Single splenocyte suspensions were prepared in Dulbecco,s Modified Eagle Medium (DMEM, Gibco) supplemented with 10% heat-inactivated fetal calf serum (Gibco) and 50 μg/ml penicillin-streptomycin (Sigma), and seeded (1 × 106 cells per well) in 96-well PVDF plates that were pre-coated with different anti-cytokine antibodies. The cells were cultured at the presence or absence of H. pylori antigens (10 μg/ml) for 40 h at 37°C and 5% CO2. The positive spots were developed following the ELISpot kits operation guide and counted by an Elispot Reader (S5VERSA, CTL, Cleveland, OH, USA). The number of ELISpot positive splenic lymphocytes was numerated and presented, respectively, for IFN-γ (A), IL-17 (B) and IL-4 (C). Data are representative of one of two independent experiments giving similar results. The bars represent mean ± SEM of the positive cells to the respective cytokines