Figures & data
Table 1. Baseline characteristics of infants participating in this study in China, 2018
Figure 1. Profile of the clinical trial
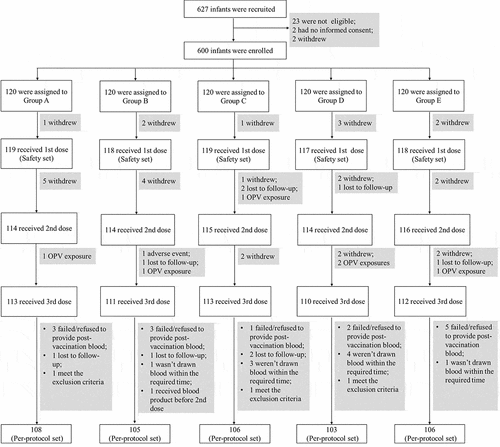
Table 2. Seropositivity rates, seroconversion rates, GMTs and GMFIs of poliovirus type-specific neutralizing antibody in infants after three doses of vaccine in China, 2018
Figure 2. Distribution of post-vaccination antibody titers
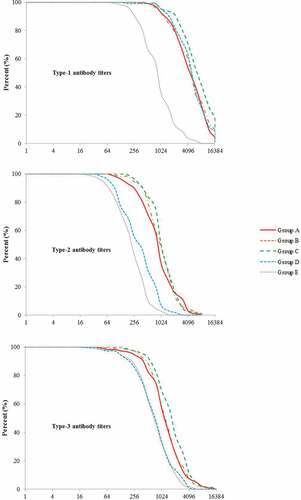
Table 3. Overall adverse events in infants after three doses of vaccine in this study in China, 2018
Table 4. Concomitant vaccination of the trial participants in this study in China, 2018
