Figures & data
Figure 1. Design and in-vitro testing of scFv-Fc RSV-F dMAb construct: (a) Illustration of human IgG1 and scFv-Fc formats. The complete hinge and Fc portions of the molecule are retained in the scFv-Fc format. Yellow line connecting VH and VL of the scFv-Fc molecule represents the (G4 S) 3 linker (b) Plasmid map of RSV-F dMAb (bGH PolyA – bovine growth hormone polyadenylation signal, KanR – kanamycin resistance gene, pUC ori – pUC origin of replication, hCMV promotor – human cytomegalovirus promotor) (c) In vitro expression of RSV-F dMAb. Immuno-fluorescence staining was performed on HEK293 T cells 3 days after in vitro transfection (DAPI – blue, RSV-F dMAb – red) (d) Western-blot analysis of cell-culture supernatant of RSV-F dMAb in vitro transfected HEK293 T cells harvested on day 3 post-transfection (lane 1: IgG-RSV dMAb, lane 2: scFv-Fc RSV-F dMAb).
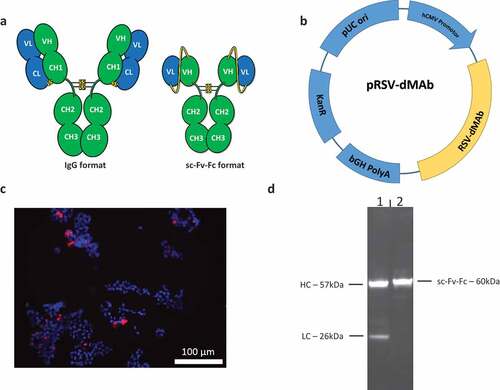
Figure 2. Schematic of RSV-F dMAb in-vivo delivery platform: (a) In vivo EP facilitates the delivery of the dMAb to the muscle tissue, myocytes express (b) and secret the encoded mAb (c). mAb is distributed systemically. (b) Example of expression of dMAb in myocytes 3 days after delivery of 50 µg dMAb or pVax (empty expression vector control) to mouse TA (DAPI – blue, dMAb – green). (c) Serum concentration of RSV-F dMAb and Palivizumab after delivery of 200 µg RSV-F dMAb plasmid or 15 mg/kg (clinical dose) Palivizumab measured by ELISA (± SEM, n = 6) dotted line indicates the level of protection (20 µg/ml) for Palivizumab.
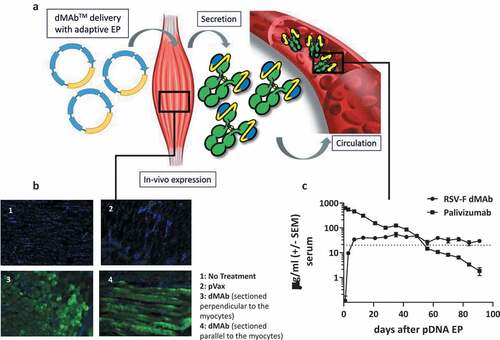
Figure 3. In-vivo expression of RSV-F dMAb in BALB/c mice: 200 µg RSV-F dMAb plasmid was administered IM in BALB/c mice (a-d). Serum samples were taken from treated mice before treatment (day 0) and 7 days after treatment to measure (a) Serum expression of RSV-F dMAb. scFv-Fc construct (± SEM, n = 5–8) (b) RSV-F antigen binding of dMAb. RSV-F binding signal of serum samples from treated (squares) and naïve (triangles) mice (± SEM, n = 4) (c) In vitro neutralization of RSV-A virus. Neutralization titer of serum samples from treated (squares) or naïve (triangles) mice (log2 serum dilution of 60% reduction of plaque-formation; ±SEM, n = 3–11, dotted line indicates LOD at serum-dilution of 1/20) (d) Concentration of scFv-Fc RSV-F dMAb in BAL samples 7 days after RSV-dMAb pDNA delivery in treated (squares) or naïve (triangles) mice (mol scFv-Fc dMAb per gram total protein in lavage sample, n(naïve) = 2, n(RSV-F dMAb) = 3).
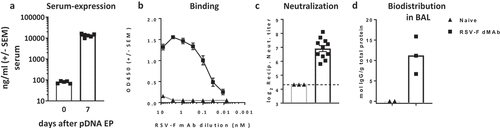
Figure 4. Characterization of RSV-F dMAb in cotton rats: (a) Local expression of dMAb demonstrated by Immuno-fluorescence staining of RSV-F dMAb in cotton rat TA muscle tissue 7 days after delivery of 400 µg RSV-F dMAb pDNA to the tissue site (DAPI – blue; RSV-F dMAb – green; sectioned perpendicular to myocytes) (b) Levels of RSV-F dMAb (ng/ml) was measured for 39 days after IM administration of 800 µg dMAb-plasmid (±SEM, n = 5). (c) Virus neutralization function of in-vivo expressed RSV-F dMAb. Serum samples were harvested and tested 7 days after delivery of 2.4 mg scFv-Fc dMAb-pDNA: neutralizing titer (±SEM, dotted line indicates LOD at serum-dilution of 1/20). (d) Concentration of RSV-F dMAb in BAL samples from treated cotton rats.
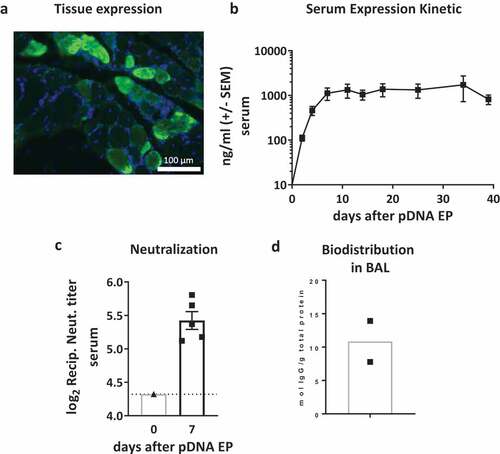
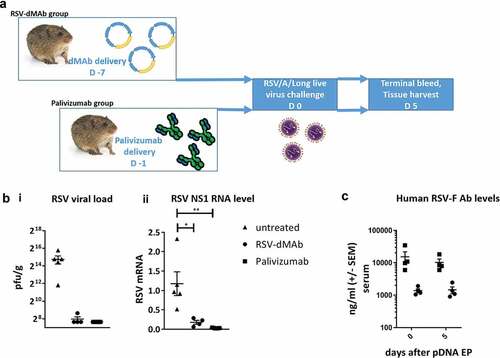
Figure 5. RSV-F dMAb confers protection against LRD after RSV/A challenge of cotton rats: (a) Schematic of cotton rat challenge study: Animals were treated with 2.4 mg RSV-dMAb 7 days before challenge or with an IM injection of 15 mg/kg Palivizumab 1 day before challenge (b) Viral load of cotton rat lung tissue (pfu/g) harvested 5 days after intra-nasal live virus challenge with RSV/A/long (± SEM n = 4–5) (i). RSV Nonstructural protein-1 (NS-1) mRNA levels (log2 and normalized to beta-Actin) of cotton rat lung tissue 5 days after intra-nasal live virus challenge with RSV/A/long (± SEM, n = 4–5, Mann–Whitney non-parametric t-test: p (untreated vs RSV-dMAb) = 0.0159; p (untreated vs Palivizumab) = 0.0079) (ii). (c) Serum levels of Palivizumab and scFv-Fc RSV-F dMAb in cotton rats at the day of challenge (day 0) and 5 days after challenge (± SEM, n = 4–5).