Figures & data
Table 1. Proliferative response and cytokine production by spleen cells following two doses of F-HA vs. CCS/C-HA vaccination in WT, Tlr2-/-, and Tlr4-/- mice
Table 2. Proliferative response and cytokine production by spleen cells following vaccination with one or two doses of F-HA vs. CCS/C-HA vaccine in WT and Myd88-/- mice
Figure 1. Effect of mouse strain and gender on immunogenicity of F-HA vs. CCS/C-HA
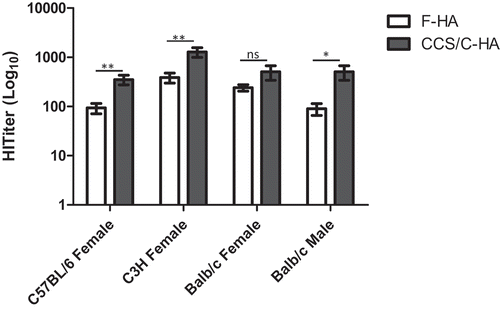
Figure 2. TLR2 and TLR4 do not contribute to antiviral immunity induced by F-HA and CCS/C-HA
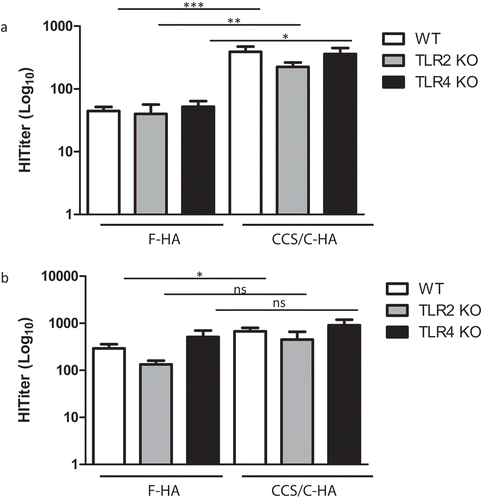
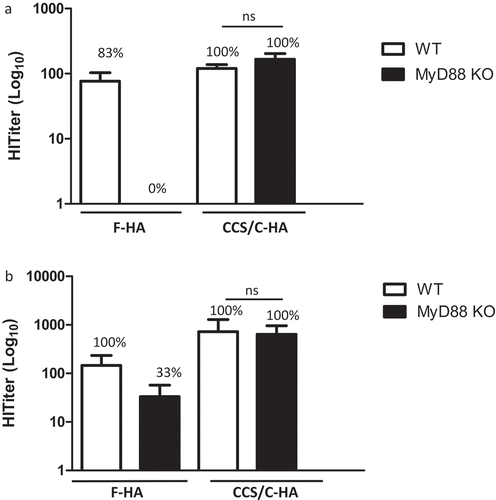
Figure 3. Adjuvant effect of CCS/C-HA does not require MyD88