Figures & data
Figure 1. TMV vaccine preparation and characterization from 4T1 tumor tissue. (a) Zetasizer analysis of TMVs, (b) Size of 4T1 TNBC TMVs, (c) Yield of TMV, (d) Scanning electron microscopy (SEM) of TMV from 4T1 breast cancer tumor tissue, (e) Schematics of incorporating GPI-ISMs onto TMVs by protein transfer approach and (f) Flow cytometry analysis of TMV vaccine showing protein transfer-mediated incorporation of GPI-B7-1 and GPI-IL-12 onto 4T1 TMV
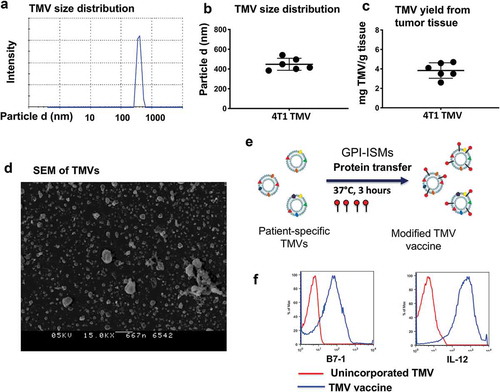
Figure 2. Co-administration of anti-CTLA-4 mAb with immunotherapy increases key immune mediators in serum. (a) Immunotherapy protocol design. BALB/c mice (n = 5) were immunized three times 14 d apart with PBS, anti-CTLA-4 mAb, TMV vaccine, or TMV vaccine + anti-CTLA-4 mAb. Serum was collected and pooled on d 5 after the final immunization. (b) eBioscience 36-Plex Luminex assay was performed by Charles River Laboratories. The values were an average of duplicate readings
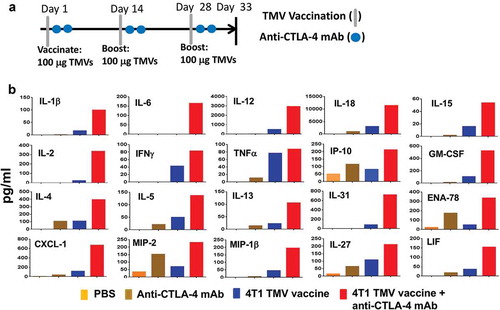
Figure 3. A combination of TMV vaccine and anti-CTLA-4 mAb enhances the survival of mice after resection of primary tumor. (a) Tumor challenge/resection and immunotherapy protocol design. (b) Kaplan-Meier survival curves of control and anti-CTLA-4 mAb treated groups. Comparison of TMV vaccine + anti-CTLA-4 mAb and anti-CTLA-4 mAb alone yields p = .0074, n = 5–10. Comparison of TMV vaccine + anti-CTLA-4 mAb and TMV vaccine + Mouse IgG yields p = .0105, n = 5–10. Log-rank (Mantel-Cox) test used for comparison analysis. (c) Kaplan-Meier survival curves of control and anti-PD-L1 mAb treated groups. **p ≤ 0.01.
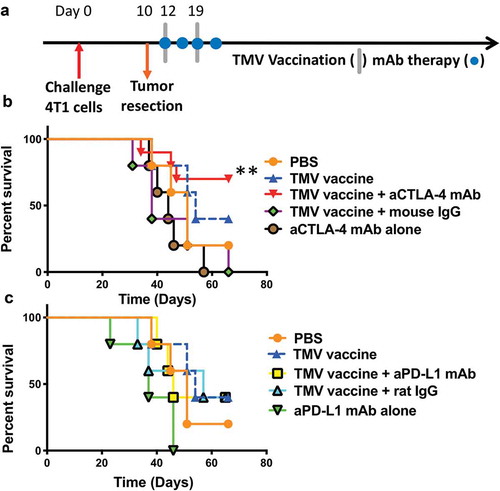
Figure 4. Reduction in metastasis to the lung in mice receiving a combination of TMV vaccine and anti-CTLA-4 mAb. (a) Tumor challenge, resection, and immunotherapy protocol design. (b) Metastasis evaluated using a clonogenic assay at d 35–36 post orthotopic challenge. Statistical differences were calculated using Mann–Whitney U test *p < .05, n = 4–5.
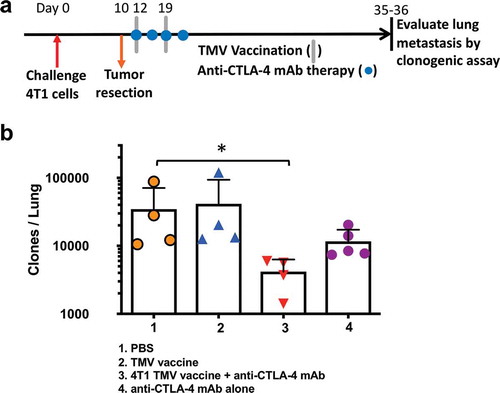
Figure 5. The depletion of CD8 T cells abrogates the impact of immunotherapy on 4T1 metastasis. (a) Immunotherapy, cell depletion, and tumor challenge protocol design. (b) Metastasis evaluated using a clonogenic assay at d 20 post orthotopic challenge with 4T1 cells. Statistical differences were calculated using t-test. **p < .01, n = 4–5.
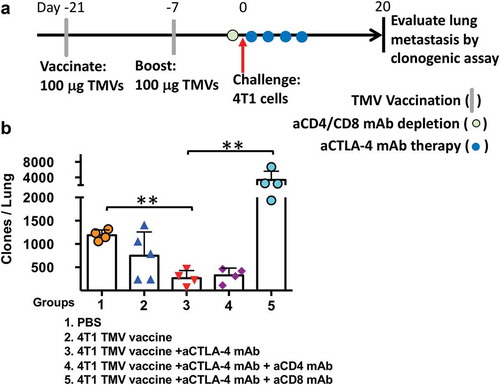
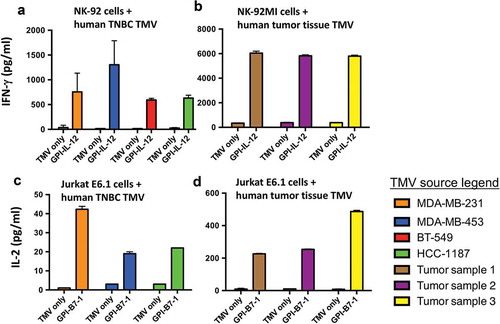
Figure 6. Human GPI-ISMs incorporated into TMVs prepared from human TNBC cell lines or breast cancer tumor tissues are able to stimulate PBMC, NK-92, and Jurkat E6.1 cells. (a) Human NK-92 cells and (b) human NK-92MI cells (2 x 105/ml) were stimulated with TMVs containing incorporated human GPI-IL-12 (40 μg/ml TMV) from various human TNBC cell lines (a) or patient-derived breast cancer tumor tissue (b) for 48 h. Supernatant was collected and IFN-γ was measured by ELISA. Human Jurkat E6.1 cells (6 x 105/ml) were stimulated with TMVs with/without GPI-B7-1 (40 μg/ml TMV) from various human TNBC cell lines (c) or patient-derived breast cancer tumor tissue (d) in combination with anti-human CD3 mAb for 24 h. Supernatant was collected and IL-2 was measured by ELISA. IL-2 levels were undetectable in Jurkat E6.1 with anti-CD3 alone. Values were average of triplicates and represented as Mean ± SD