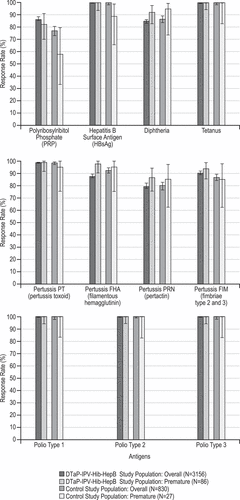
Figures & data
Table 1. Premature infant accounting*
Table 2. Analysis of the clinical adverse events after any dose of vaccine in the overall and premature populations for DTaP-IPV-Hib-HepB and control vaccines
Figure 1. Selected systemic and injection-site adverse events days 1 to 15 following any dose of vaccination
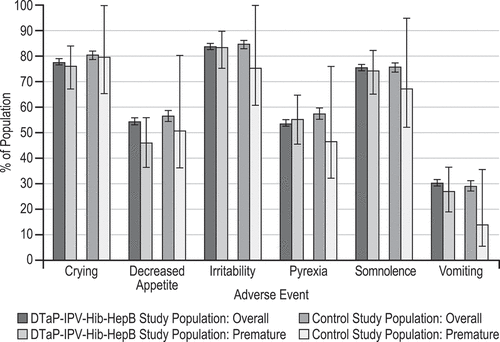
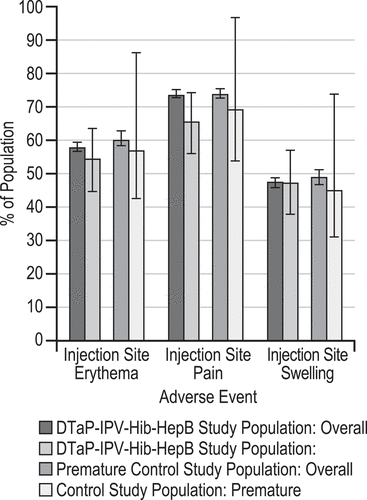
Figure 2. Selected injection-site ae days 1 to 15 following any dose vaccination