Figures & data
Figure 1. HBV virus amplification & characterization from HepG2.2.15 cells.
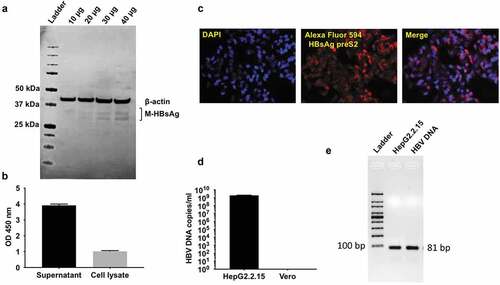
Figure 2. HBV-DMAb expression in vitro and in vivo.
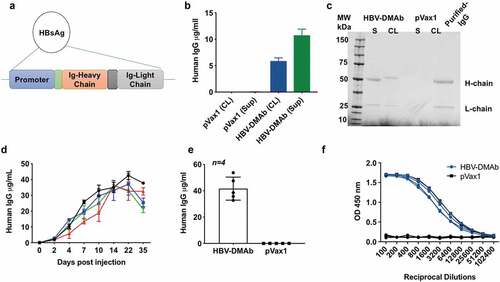
Figure 3. HBV-DMAb binds a specific epitope of HBsAg.
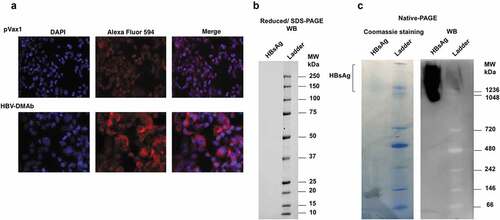
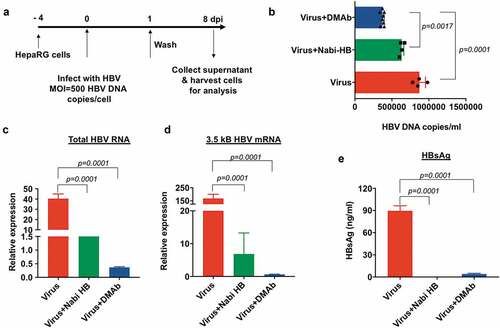
Figure 4. HBV-DMAb neutralizes HBV and blocks infection of HepaRG cells.