Figures & data
Figure 1. Dose-optimization and efficacy studies using the 2012–2013 influenza vaccine formulations in cotton rats. (a,b) Dose–range study of immunogenicity of FluLaval 2012–2013 during the corresponding vaccine season (VSeason: 2012–2013). Young animals were immunized intramuscularly (i.m.) twice (with 3 weeks in between) with the indicated doses of FluLaval or infected intranasally (i.n.) once with influenza A/Victoria (H3N2) (Vict) and blood was collected for analysis of serum HAI titers (a) or binding IgG (b) against FluLaval 2012–2013 three weeks after the last immunization or 6 weeks after A/Victoria infection. Results represent GMT±SE for 8–10 animals per group. Negative control animals (Ctr) were unimmunized and uninfected. (c) Efficacy of FluLaval 2012–2013 and FluMist 2012–2013 in the young and aged cotton rats. Animals immunized once with FluLaval or FluMist were infected i.n. with A/California (A/Cal) three weeks later and sacrificed 1 day later for analysis of influenza load in the lung by qPCR. Control animals were infected with A/Cal and re-infected 3 weeks later (secondary infection, °2) or mock immunized and infected with A/Cal (primary infection, °1). Results represent GMT±SE for 5 animals per group. *p< .05 when compared to influenza M mRNA level in the same age animals with primary infection. No significant differences were found between influenza M mRNA levels in animals of different ages treated the same way
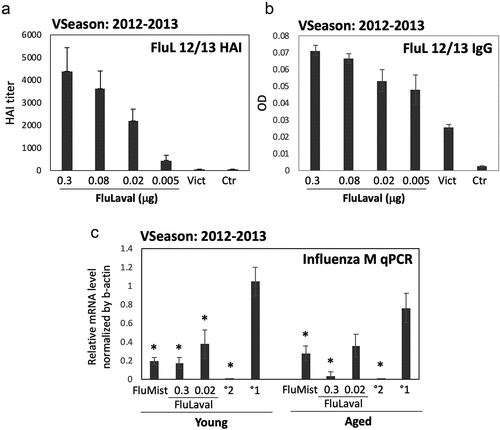
Figure 2. Antiviral efficacy of FluLaval 2016–2017 in the young and aged cotton rats when given as a two-times (a) vs. one-time (b) immunization. (a) Two-times vaccination. Young and aged cotton rats were immunized with the indicated doses of FluLaval (FluL) 2016–2017 and boosted 3 weeks later. After another 3 weeks animals were infected i.n. with influenza A/California (A/Cal) at 107 TCID50 per 100 g animal and sacrificed one day later for analysis of viral load in the lung and nose. Results of viral titration by TCID50 assay (VT) are shown. Control animals were infected i.n. with A/Cal and re-infected 6 weeks later (°2) or mock immunized and infected with A/Cal (°1). (b) One-time vaccination. Young and aged cotton rats were immunized with FluLaval once, infected i.n. with A/Cal 3 weeks later, and sacrificed 1 day after infection. Control animals were mock-immunized with PBS and infected i.n. with A/Cal once (°1) or twice with an interval of 3 weeks (°2) and sacrificed one day after challenge. Unimmunized and uninfected animals were included as additional controls (“-“). Results represent GMT±SE for 5–6 animals per group. *p < .05 when compared to primary infection of the same age animals, #p < .05 when compared to the same treatment, opposite age
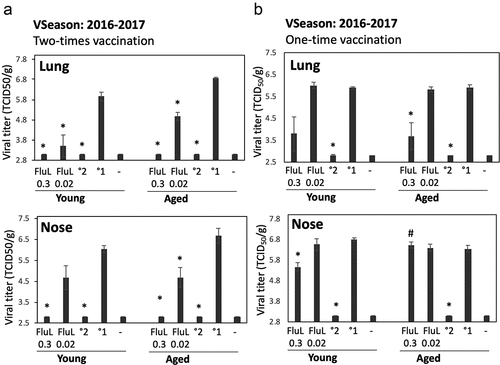
Figure 3. Immunogenicity of seasonal influenza vaccines in the young and aged cotton rats. (a) Immunogenicity of FluLaval 2012–2013 and FluMist 2012–2013 in the young and aged cotton rats. Animals were immunized with the indicated doses of FluLaval 2012–2013 i.m. or with FluMist 2012–2013 i.n. during the corresponding vaccine season and boosted 3 weeks later. Control animals were infected i.n. with influenza A/California (Cal). Serum samples were collected prior to boost and three weeks after the boost for analysis of HAI titers against A/Cal. Results represent GMT±SE for 11–15 animals per group. Negative control animals (Ctr) were unimmunized and uninfected. (b,c) Immunogenicity of FluLaval (FluL) 2016–2017 in the young and aged cotton rats. Animals were immunized as described above but using the FluLaval 2016–2017 formulation during the corresponding vaccine season. Control animals were infected with influenza A/California or left unimmunized and uninfected (Ctr). Blood samples were collected for analysis of serum HAI titers against A/Cal (b) or FluLaval 2016–2017 (c). Results represent GMT±SE for 9–12 animals per group (except for Ctr group, where 5 samples were included). *p< .05 when compared to titers in the same age animals with primary infection, same day; & p< .05 when compared to d21 samples collected after the same dose vaccination for the same age animals; # p< .05 when compared to young animals, same vaccine, dose, and day of collection; ⊓ (connector) p< .05 when compared to the lower vaccine dose in the same age animals, same day of blood collection
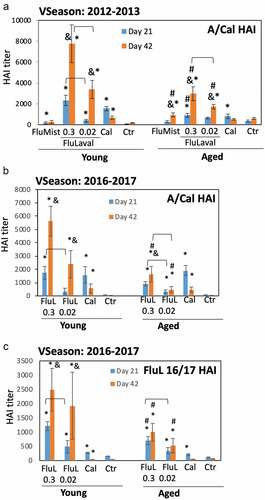
Table 1. Seroconversion of young and aged animals by FluLaval 2016–2017. Seroconversion was defined as an increase in HAI GMT of fourfold or higher over the lower limit of assay (HAI = 40). Percentage of seroconverted animals (if greater than 0) is given in parenthesis
Figure 4. Lung histopathology in the young and aged animals immunized with FluLaval 2016–2017 twice (a) or once (b). Animals were immunized or infected as described in the legend to and sacrificed 1 or 4 days after infection for analysis of pulmonary histopathology. (a) Pulmonary histopathology in influenza-infected animals after two-times immunization with FluLaval or 6 weeks after the initial infection with A/Cal. (b) Pulmonary histopathology in influenza-infected animals after one-time immunization with FluLaval or 3 weeks after the initial infection with A/Cal. Results represent GMT±SE for 5–6 animals per group. PB: peribronchiolitis, PV: perivasculitis, IP: interstitial pneumonitis, A: alveolitis. * p < .05: a decrease compared to primary infection of the same age animals; x p < .05: a decrease compared to primary infection of the same age animals; Δ p < .05: an increase compared to primary infection of the same age animals; @ p < .05: an increase compared to primary infection of the same age animals
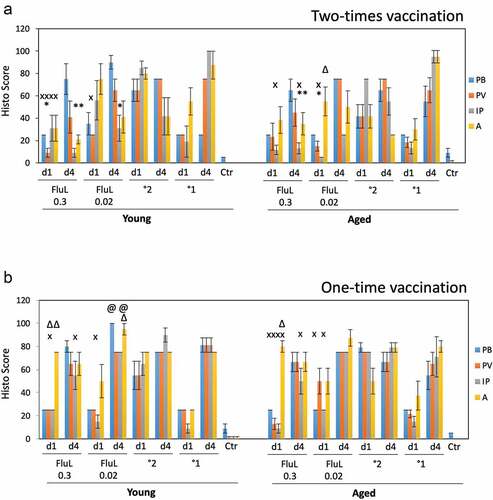
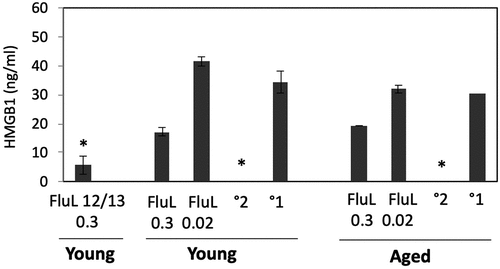
Figure 5. Serum HMGB1 levels in cotton rats immunized with FluLaval and challenged with A/California. Young and aged animals were immunized with the indicated doses of FluLaval 2016–2017 (FluL) twice with an interval of 3 weeks and infected with influenza A/Cal 3 weeks after the second immunization. Control animals were infected with A/Cal and re-infected 6 weeks later (secondary infection, °2) or mock immunized and infected with A/Cal (primary infection, °1). Serum from a group of young animals immunized in a similar manner with FluLaval 2012–2013 (FluL 12/13) and challenged with A/Cal was included in the analysis. Serum was obtained on day 4 post-challenge. Results represent GMT±SE for 5–6 animals per group. *p < .05 when compared to HMGB1 levels in serum of the same-age animals with primary infection