Figures & data
Table 1. Comparison of demographic and socioeconomic characteristics, using Chi-square tests, between the children included and children excluded from this study, Anhua county, Hunan province, southern China
Table 2. Vaccination coverage and timeliness for EPI vaccines, by dose. Vaccines scheduled at <12 months are shaded in gray
Table 3. Vaccination coverage and timeliness for non-EPI vaccines, by dose, based on the study population (rural children) in Anhua county, Hunan province, southern China
Figure 1. Vaccination coverages of EPI vaccines by birth cohort. No data was collected for a 2013 cohort in the original sero-survey study. Each line begins from the cohort in which the vaccine was first introduced to the EPI, and ends with the cohort for which the vaccine is not age-appropriate
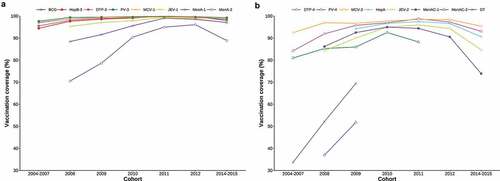
Figure 2. Vaccination coverage of non-EPI vaccines by birth cohort. No data was collected for a 2013 cohort in the original sero-survey study. PCV7 and ORV-3 are omitted due to negligible vaccine coverage across all cohorts
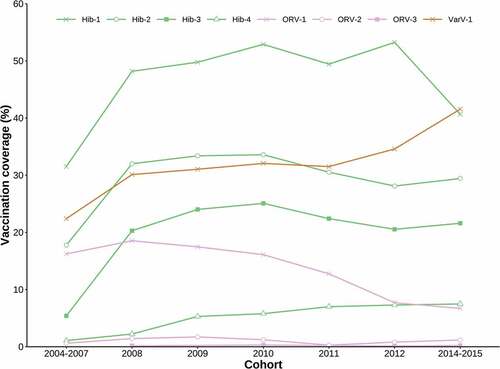
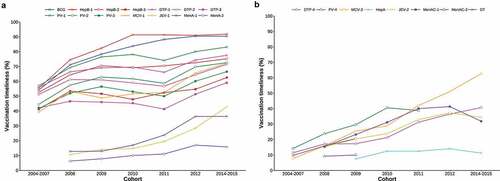
Figure 3. Vaccination timeliness for EPI vaccines, by birth cohort. No data was collected for a 2013 cohort in the original sero-survey study. Each line begins from the cohort in which the vaccine was first introduced to the EPI, and ends with the cohort for which the vaccine is not age-appropriate