Figures & data
Table 1. Summary of baseline characteristics and solicited local/systemic events
Figure 1. Detection of SeV or RSV F protien sequence by PCR
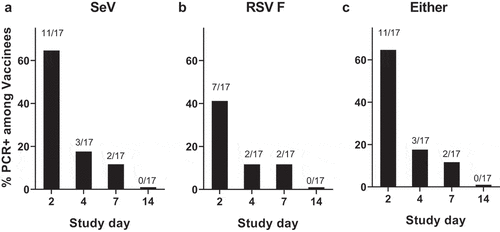
Table 2. Antibody response by treatment group in the modified intent-to-treat population
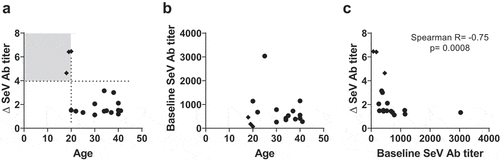
Figure 2. Antibody responses toward SeV and RSV F