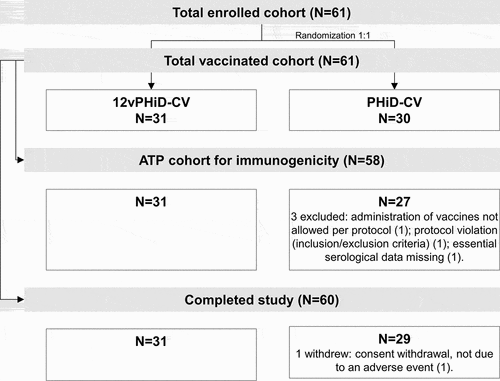
Figures & data
Table 1. Demographic characteristics for study participants (total vaccinated cohort)
Figure 2. Percentage of toddlers with solicited local (A) and general (B) adverse events within the 7-day period following vaccination (total vaccinated cohort)
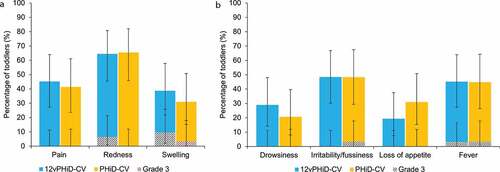
Table 2. Percentages of toddlers with pneumococcal serotype-specific antibody concentrations ≥0.2 µg/mL and antibody GMCs, by timepoint (according-to-protocol cohort for immunogenicity)
Table 3. Percentages of toddlers with pneumococcal serotype-specific OPA titers above or equal to the assay serotype-specific LLOQ and OPA GMTs, by timepoint (according-to-protocol cohort for immunogenicity)
Table 4. Percentages of toddlers with anti-protein D antibody concentrations ≥100 EL.U/mL and antibody GMCs, by timepoint (according-to-protocol cohort for immunogenicity)