Figures & data
Figure 1. Time schedule for HRV and HBRV vaccination according to the respective SmPC.Citation9,Citation10
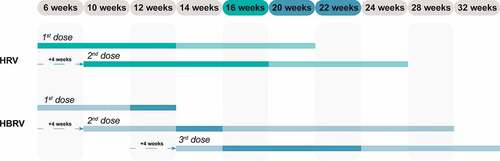
Figure 2. Distribution by infant age (weeks) of hospitalization rates for intussusception in children below 2 years of age in Italy (modified fromCitation26)
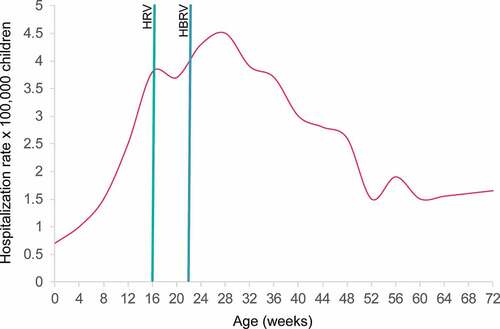
Table 1. Compliance to vaccination schedule (% per dose).Citation31
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this research.

