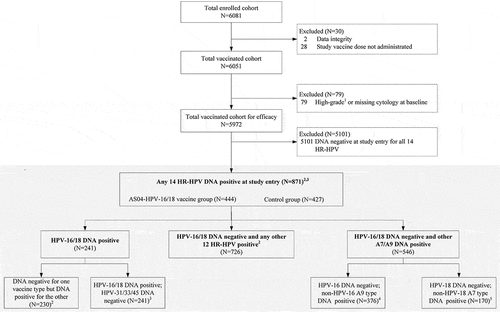
This post-hoc analysis focused on women with existing HR-HPV infection (DNA-positive) at baseline, irrespective of serostatus. For VE against individual HPV type, subjects were DNA-negative to the corresponding HPV type at baseline. For VE against combined types, subjects were DNA-negative to at least one HPV type at baseline (subjects were in the analysis of at least one single type). Besides meeting the above criteria for baseline status, subjects included in the relevant analyses must have sample available after vaccine dose 1 for the evaluation of the virological endpoints, including incident, 6-month and 12-month HPV persistent infections.
Citation1High-grade cytology included squamous cells cannot exclude high-grade squamous intraepithelial lesions, high-grade squamous intraepithelial lesions, atypical glandular cells, or malignancy
Citation2VE against incident, 6-month and 12-month persistent infections associated with HPV-16, HPV-18 and HPV-16 and/or HPV-18 was assessed.
Citation3VE against incident, 6-month and 12-month persistent infections associated with HPV-31, HPV-33, HPV-45, and HPV-31 and/or HPV-33 and/or HPV-45 was assessed.
Citation4VE against incident, 6-month and 12-month persistent infections only associated with HPV-16 were assessed.
Citation5VE against incident, 6-month and 12-month persistent infections only associated with HPV-18 were assessed. HR-HPV, high-risk human papillomavirus; N, number of subjects; VE: vaccine efficacy.