Figures & data
Table 1. Participant baseline characteristics: randomized cohorts, Part A and Part B
Table 2. Summary of treatment-emergent adverse events
Figure 3. Solicited Injection-Site Adverse Events
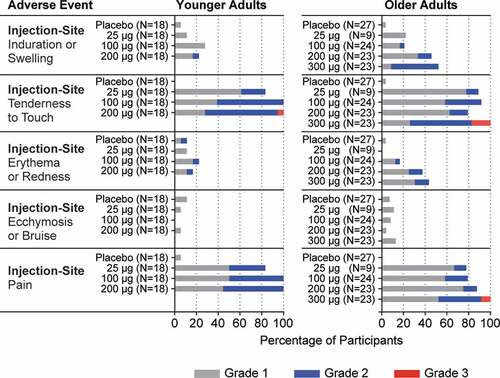
Figure 4. Solicited Systemic Adverse Events
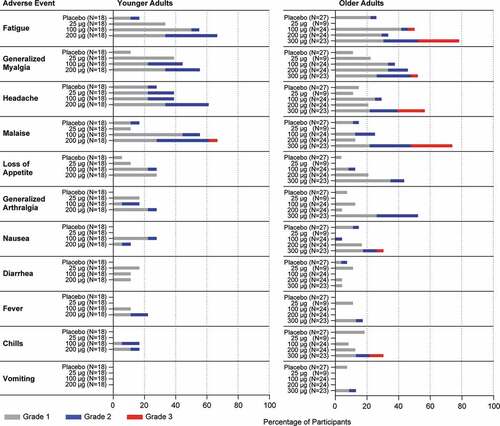
Figure 5. Serum Neutralization Titers against RSV A and RSV B
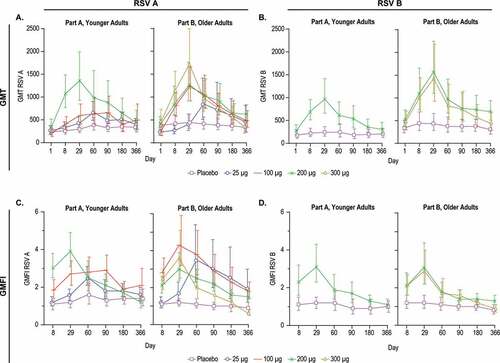
Figure 6. Serum Antibody Titers to Prefusion F Protein
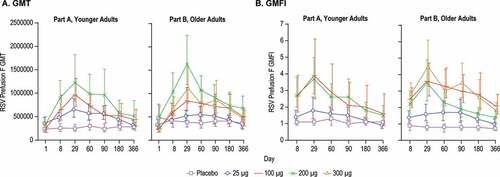
Figure 7. D25 and Palivizumab Competing Antibodies
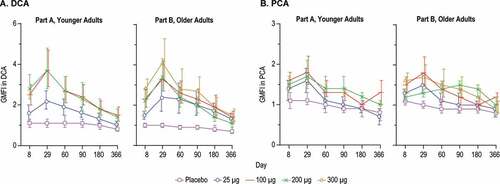
Figure 8. IFN-γ ELISPOT
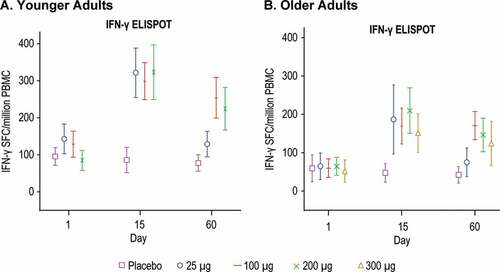
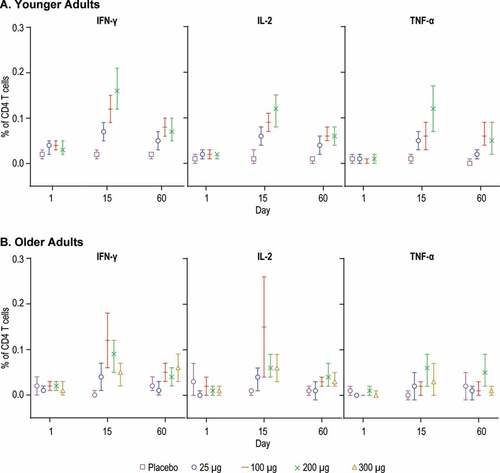
Figure 9. CD4 T cell-responses by Intracellular Cytokine Staining