Figures & data
Figure 1. Disposition of study participants
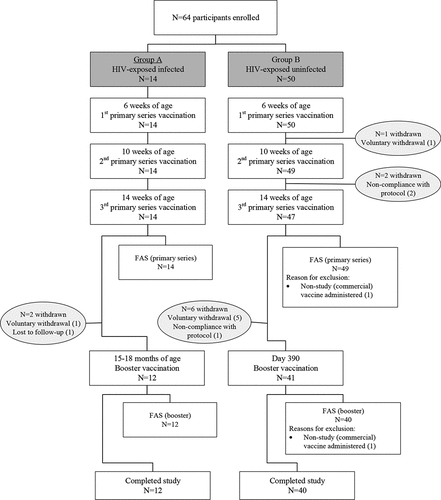
Table 1. Seroprotection rates, seroconversion rates, and vaccine response rates pre- and post-primary series and booster vaccination (FAS)
Table 2. Geometric mean concentrations and titers pre- and post-primary series and booster vaccination (FAS)
Table 3. Immediate, solicited, unsolicited, and serious adverse events during the study (SS)
