Figures & data
Table 1. Meningococcal conjugate and recombinant protein vaccines used globally
Figure 1. Countries with recent MenACWY vaccine recommendations.Citation31,Citation37–59
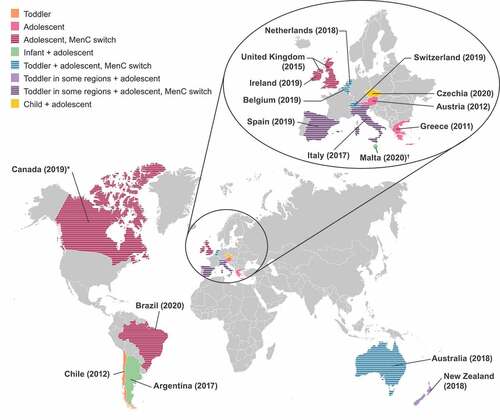
Table 2. Overview of included studies
Figure 2. Serum bactericidal antibody measurements against serogroup C at 1 month postprimary and postbooster vaccination in infants vaccinated with MenACWY-TT, MenC-TT, or MenC-CRM197/Al(OH)3 at 2, 4, and 12 months of age.Citation60 (a) Percentage of infants with rSBA titers ≥1:8, (b) rSBA GMTs, (c) percentage of infants with hSBA titers ≥1:8, and (d) hSBA GMTs.
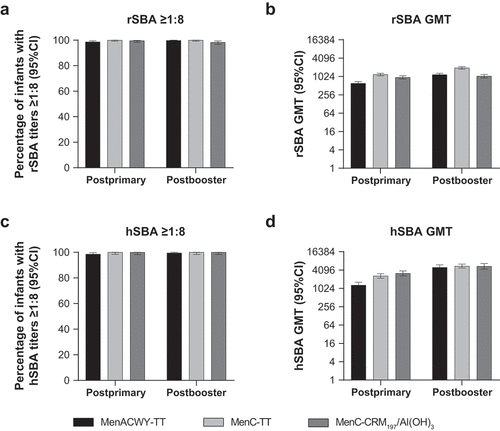
Figure 3. Serum bactericidal antibody measurements against serogroup C at 1 month/42 days postvaccination in toddlers administered a single dose of MenACWY-TT or MenC-CRM197/AlPO4 vaccine.Citation61–63,Citation67 (a) Percentage of toddlers with rSBA titers ≥1:8, (b) rSBA GMTs, (c) percentage of toddlers with hSBA titers ≥1:8, and (d) hSBA GMTs.
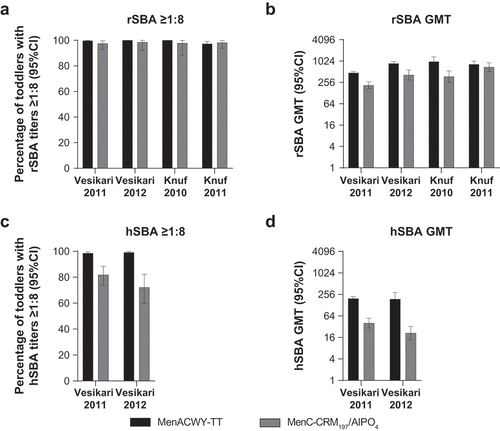
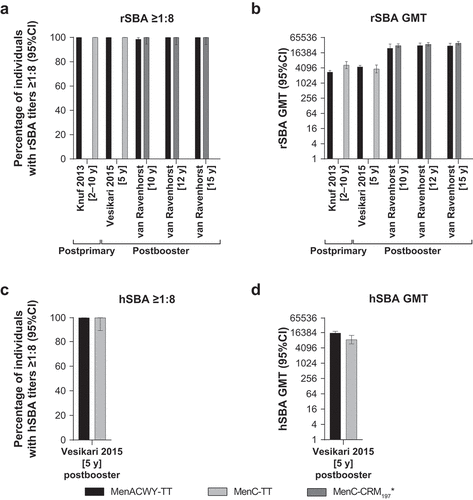
Figure 4. Serum bactericidal antibody measurements against serogroup C at 1 month postvaccination in individuals aged ≥2 years administered a single or booster dose of MenACWY-TT, MenC-CRM197/Al(OH)3, MenC-TT, or MenC-CRM197/AlPO4 vaccine.Citation64–66 (a) Percentage of individuals with rSBA titers ≥1:8, (b) rSBA GMTs, (c) percentage of individuals with hSBA titers ≥1:8, and (d) hSBA GMTs.