Figures & data
Table 1. Demographic characteristics of participants (total vaccinated cohort)
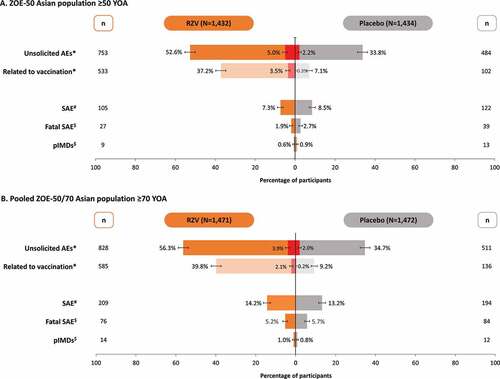
Figure 2. Vaccine efficacy against first or only episode of HZ by age and follow-up year in (A) ZOE-50 Asian population ≥50 YOA and (B) pooled ZOE-50/70 Asian population ≥70 YOA (modified total vaccinated cohort)
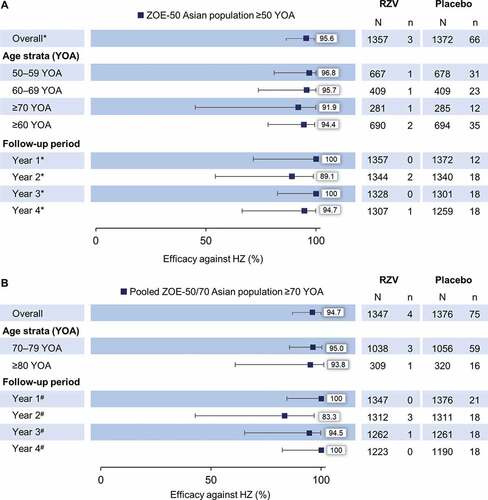
Figure 3. Solicited local and general adverse events (AEs) reported within 7 days after vaccination in (A) ZOE-50 Asian population ≥50 YOA and (B) pooled ZOE-50/70 Asian population ≥70 YOA (TVC diary card sub-cohort)
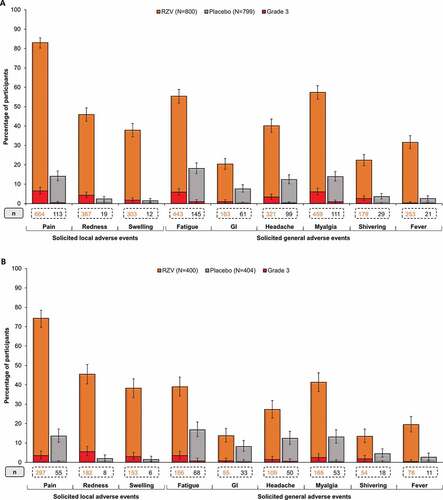
Figure 4. Unsolicited adverse events, serious adverse events, and potential immune-mediated diseases in (A) ZOE-50 Asian population ≥50 YOA and (B) pooled ZOE-50/70 Asian population ≥70 YOA (total vaccinated cohort)