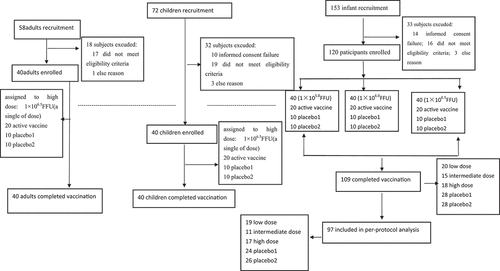
Figures & data
Table 1. Summary of demographic characteristics-adult cohort
Table 2. Summary of demographic characteristics-toddler cohort
Table 3. Summary of demographic characteristics-infant cohort
Table 4. Summary of adverse enents (AEs) –adult and toddler cohort
Table 5. Summary of adverse enents (AEs) –infant cohort
Table 6. Summary of solicited adverse enents (AEs) –infant cohort
Figure 2. Seroconversion rates in serum at 28 days after the last vaccination in vaccine and placebo groups (infants)
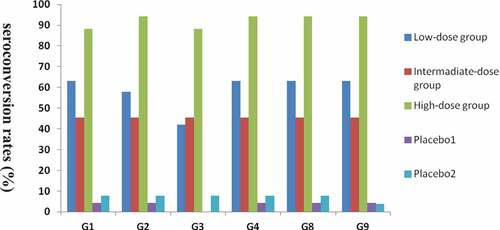
Table 7. Summary of IgA response to serotypes G1, G2, G3, G4, G8 and G9 in the per-protocol immunogenicity popolation–infant cohort