Figures & data
Table 1. Participant Demographics (Safety Population)
Figure 1. Participant Disposition. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; PCV13 = 13-valent pneumococcal conjugate vaccine; PCV20 = 20-valent pneumococcal conjugate vaccine
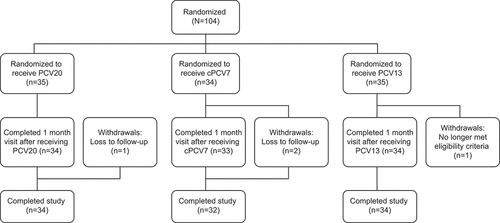
Figure 2. Reactogenicity Events Including (a) Local Reactions and (b) Systemic Events Occurring Up to 14 Days After Vaccination. The single report of fever (38.8°C; 1 participant in the PCV20 group) is not included. Number of participants: PCV20, n = 35; cPCV7, n = 34; PCV13, n = 34. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; PCV13 = 13-valent pneumococcal conjugate vaccine; PCV20 = 20-valent pneumococcal conjugate vaccine
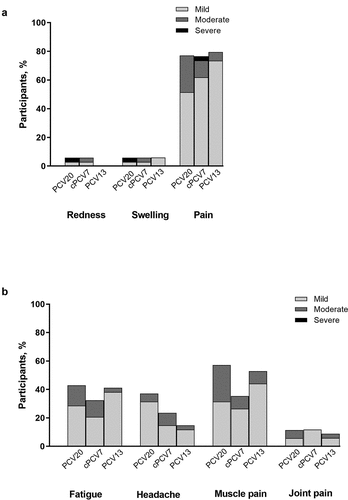
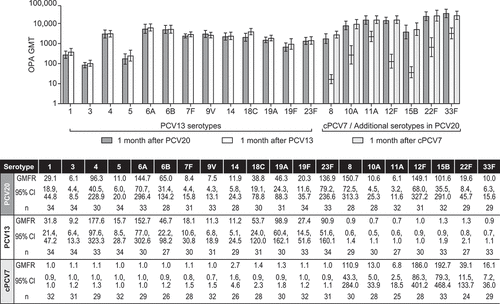
Figure 3. Pneumococcal OPA GMTs and GMFRs With 2-Sided 95% CIs for Each Serotype. Assay results below the LLOQ were set to 0.5 × LLOQ in the analysis. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMFRs = geometric mean fold-rises from before vaccination to 1 month after vaccination; GMTs = geometric mean titers; LLOQ = lower limit of quantitation; OPA = opsonophagocytic activity; PCV13 = 13-valent pneumococcal conjugate vaccine; PCV20 = 20-valent pneumococcal conjugate vaccine