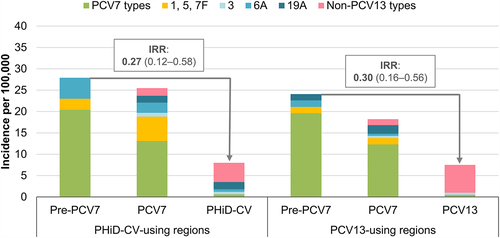
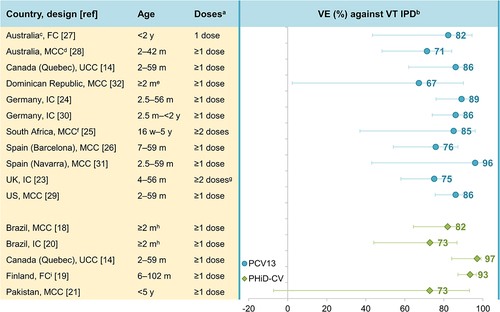
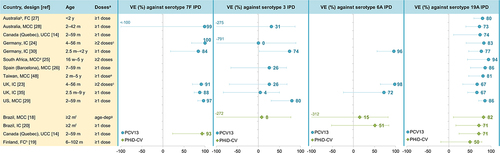
FC, full cohort; IC, indirect cohort; m, months of age; MCC, matched case-control; PCV13, 13-valent pneumococcal conjugate vaccine; PHiD-CV, pneumococcal non-typeable
Haemophilus influenzae protein D conjugate vaccine; UCC, unmatched case-control; UK, United Kingdom (England, Wales and Northern Ireland in
Citation23); US, United States; VE, vaccine effectiveness; VT IPD, vaccine-type invasive pneumococcal disease (see note
b below); w, weeks of age; y, years of age. Error bars indicate 95% confidence or credible intervals (CIs).
aEffectiveness estimates are for the indicated number of doses. Estimates for ≥1 dose were used where available.
bFor PCV13 effectiveness estimates: IPD caused by PCV13 serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F and – for references
Citation23,Citation24 – vaccine-related 6C. For PHiD-CV estimates: IPD caused by PHiD-CV serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F and – for reference
Citation14 – vaccine-related 6A.
cNon-Aboriginal children only.
dTwo different designs were used in this study to estimate VE against VT IPD: MCC: values in graph, and IC: VE was 76% (95% CI: 46–89).
eInterquartile range for age of cases: 5.3–17.4 m, and controls: 4.8–15.3 m.
fChildren not infected with human immunodeficiency virus only.
g ≥2 doses before 12 m or 1 dose on or after 12 m.
hActual age of included children was 2.6–53.1 m.
iThree different designs were used in this study: FC: values in graph, MCC: VE against VT IPD was 98% (95% CI: 90–100), and IC: VE against VT IPD was 100% (95% CI: 98–100).