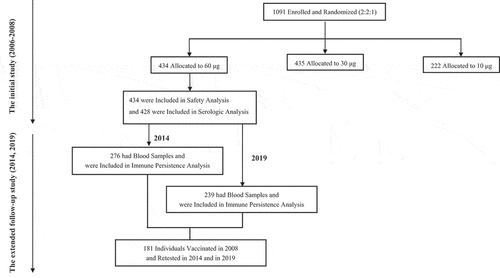
Figures & data
Table 1. Comparison of demographic characteristics between the follow-up group and the lost group in 2014 and 2019
Table 2. Seropositive rates and anti-HBs concentrations (mIU/ml) in 2008, 2014, and 2019
Table 3. Seropositive rates and anti-HBs concentrations (mIU/ml) in 181 individuals vaccinated in 2008 and retested in 2014 and in 2019
Figure 2. Classification of participants based on their serum concentration of anti-HBs antibody in 2008, 2014 and 2019. 2008 = 28 days after receiving the third dose of 60 μg HB vaccine; 2014 = 6 years after receiving the third dose of 60 μg HB vaccine; 2019 = 11 years after receiving the third dose of 60 μg HB vaccine. According to the titer of anti-HBs, the body’s response to HB vaccine can be divided into four states: <10 mIU/ml = non-responders; 10–100 mIU/ml = low-responders; 100–1000 mIU/ml = moderate responders; ≥1000 mIU/ml = high-responders
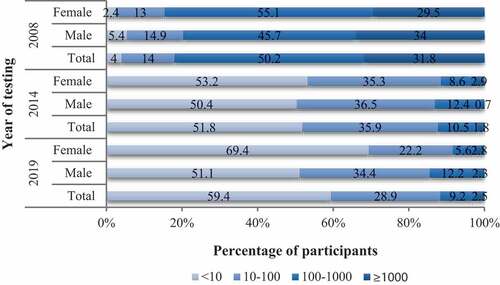
Table 4. Logistic regression analysis of seropositive rate of anti-HBs in 2019 with the baseline characters