Figures & data
Figure 1. Stage 2 subject disposition. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; PPSV23 = 23-valent pneumococcal polysaccharide vaccine
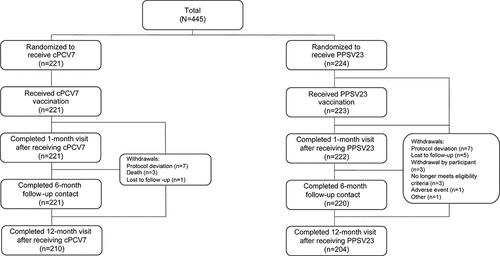
Figure 2. Reactogenicity events including (A) local reactions and (B) systemic events occurring within 14 days after vaccination (Stage 2). Three reports of fever (1 and 2 participants in cPCV7 and PPSV23 groups, respectively) are not shown. Number of participants: cPCV7, n = 221; PPSV23, n = 223. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; PPSV23 = 23-valent pneumococcal polysaccharide vaccine
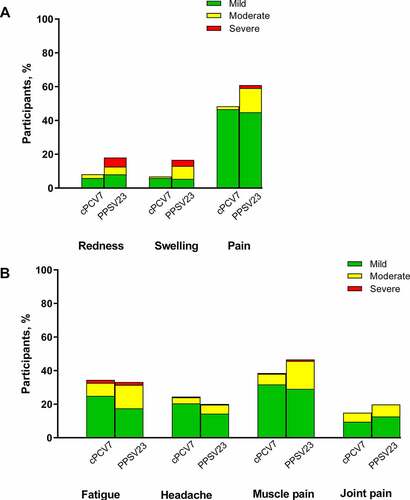
Table 1. Summary of AEs (Stage 2 Safety Population)
Figure 3. Pneumococcal OPA GMTs before and 1 month after vaccination and GMFRs 1 month after vaccination for the cPCV7 serotypes (Stage 2). The LLOQs for each serotype were as follows: 8, 16; 10A, 14; 11A, 32; 12F, 51; 15B, 36; 22F, 28; 33F, 49. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMTs = geometric mean titers; GMFRs = geometric mean fold-rises; LLOQ = lower limit of quantitation; OPA = opsonophagocytic activity; PPSV23 = 23-valent pneumococcal polysaccharide vaccine
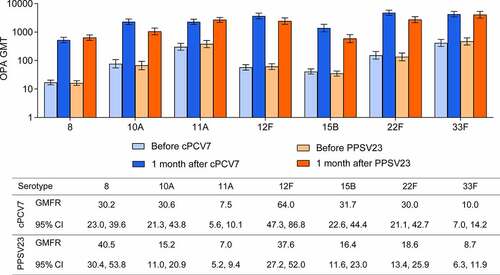
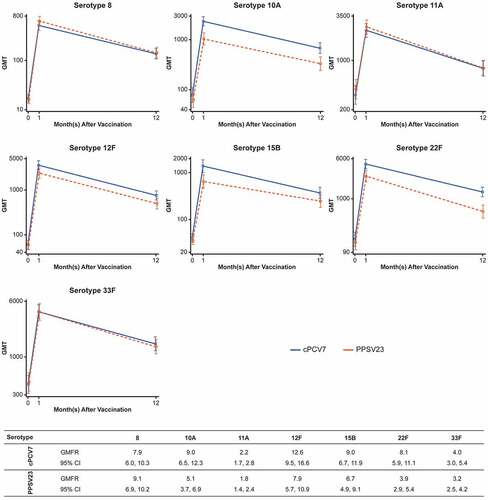
Figure 4. Pneumococcal OPA GMT antibody response curves and OPA GMFRs for the cPCV7 serotypes from baseline to 12 months after vaccination (Stage 2). Note that the y-axis scales for each graph differ. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMT = geometric mean titer; OPA = opsonophagocytic activity; PPSV23 = 23-valent pneumococcal polysaccharide vaccine