Figures & data
Figure 1. Antigen processing and presentation of Listeria monocytogenes. Lm is identified by a macrophage through pattern recognition receptors TLR2/TLR5, these initiate the MyD88 signaling pathway that leads to the activation of NF-κB, which promotes the transcription of pro-inflammatory genes and subsequent production of proinflammatory cytokines TNF-α, IL-6, and IL-12. Lm is endocytosed by the macrophage and contained in a phagosome. The phagosome fuses with a lysosome to form a phagolysosome where Lm is killed and digested, antigenic peptides derived from the digested bacteria are loaded into MHC class II molecules and presented to CD4+ Helper T cells. Alternatively, Lm has the ability to express the pore-forming listeriolysin O (LLO) toxin that perforates the phagosome and allows it to escape into the cytosol. Once in the cytosol, Lm secretes proteins that are degraded by the proteasome, these antigenic peptides are loaded into MHC class I molecules and presented to CD8+ Cytotoxic T cells. NK cells respond to IL-12 by producing IFN-γ, which enhances Cytotoxic T cell activation.Citation20
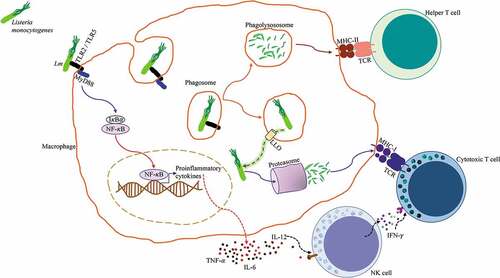
Figure 2. Antigen processing and presentation of ADXS11-001 Lm LLO-HPV E7 fusion protein
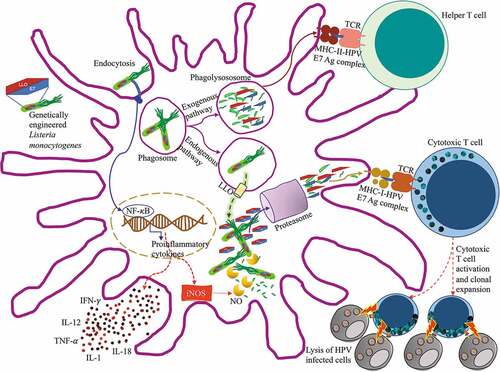
Table 1. Clinical trials with ADX11-001 in cervical cancer
Table 2. Clinical trials with ADX11-001 in HPV-associated cancers
