Figures & data
Table 1. Baseline demographics in MenACYW-TT primed and MCV4-TT primed study groups – SafAS
Figure 2. GMTs at baseline (Day 0) and Day 30 after MenACYW-TT booster dose as assessed by (a) hSBA and (b) rSBA in both MenACYW-TT primed and MCV4-TT primed study groups – PPAS.
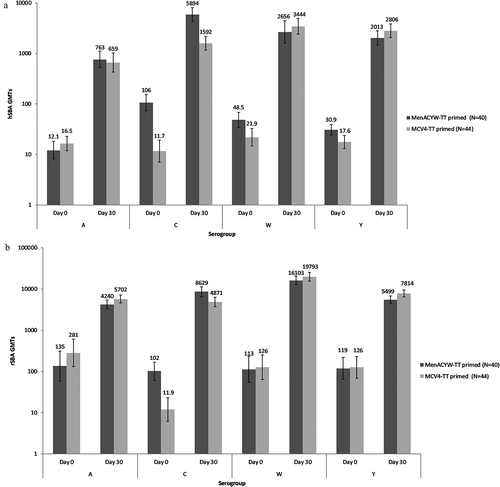
Figure 3. Proportion of participants with hSBA (a) and rSBA (b) titers ≥1:8 at pre-booster dose (Day 0) and Day 30 after MenACYW-TT booster dose in both MenACYW-TT primed and MCV4-TT primed study groups – PPAS.
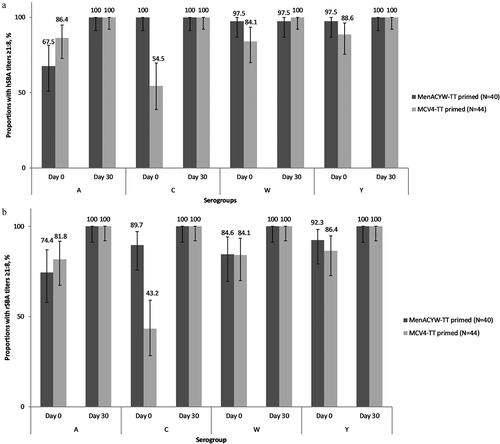
Table 2. Proportion of participants with ≥4-fold rise of hSBA and rSBA titers at Day 30 relative to baseline (Day 0) in both MenACYW-TT primed and MCV4-TT primed study groups – PPAS
Figure 4. Three-year immune persistence of primary dose and antibody response following booster dose of MenACYW-TT measured by hSBA (a) and rSBA (b) GMTs in both MenACYW-TT primed and MCV4-TT primed study groups – FASP.
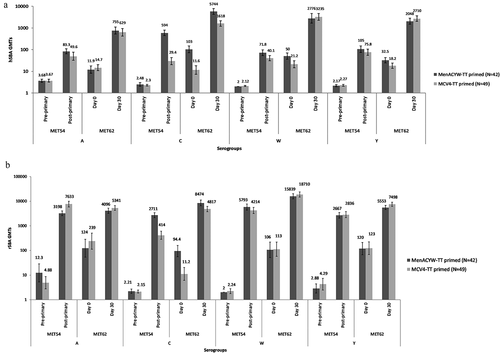
Table 3. Summary of safety outcomes from baseline (Day 0) to Day 30 after MenACYW-TT booster dose in MenACYW-TT primed and MCV4-TT primed study groups – SafAS
Supplemental Material
Download MS Word (76.7 KB)Data availability statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com