Figures & data
Table 1. Description of scenario analyses
Table 2. Number of influenza cases and influenza-related outcomes in the Canadian population aged 18–64 and 65+ with no vaccines, egg-derived vaccines only, and plant-derived QVLP vaccines only – base case results
Figure 1. Number of prevented influenza cases and influenza-related outcomes with plant-derived QVLP and egg-derived vaccines versus no vaccination in the base case analysis among the Canadian population (a) aged 18–64; and (b) aged 65+
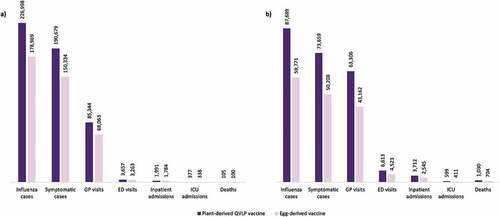
Figure 2. Number of (a) prevented cases, (b) prevented inpatient admissions, and (c) prevented deaths with plant-derived QVLP and egg-derived vaccines versus no vaccination in the base case analysis among the adult Canadian population stratified by age
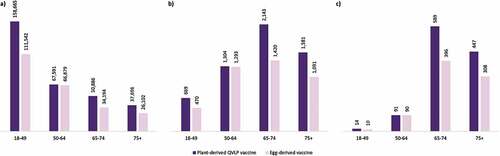
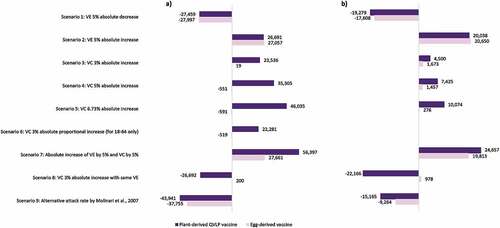
Figure 3. Difference in the number of prevented influenza cases with plant-derived QVLP and egg-derived vaccines in scenario analyses relative to respective base case results among the Canadian population (a) aged 18–64a,b and (b) aged 65+.a–c