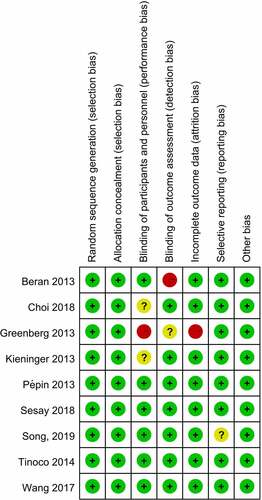
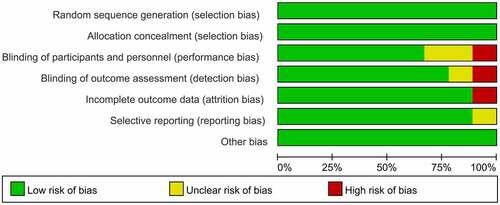
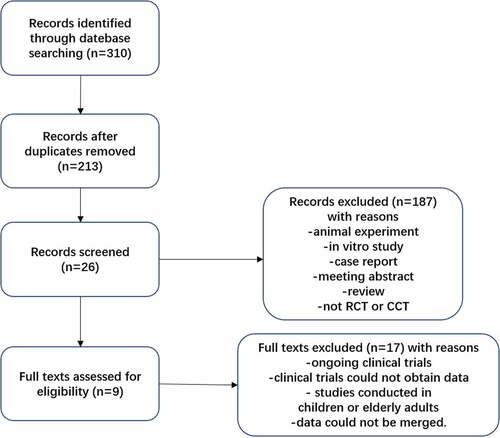
Figures & data
Table 1. Characteristics of eligible trials
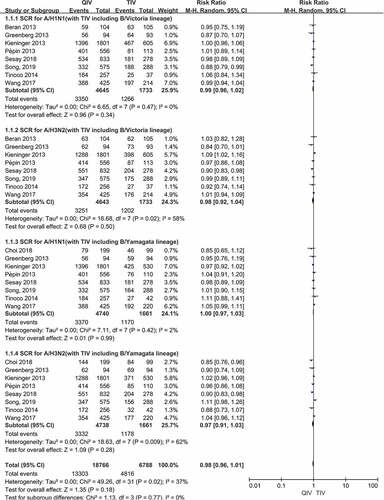
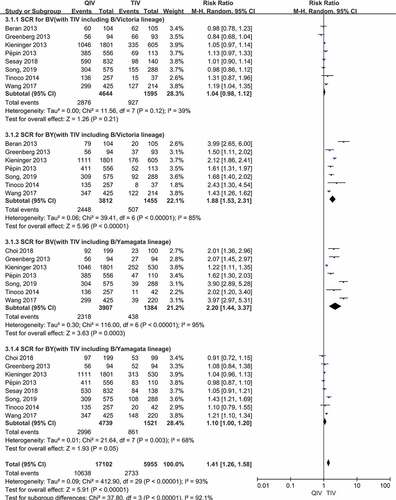
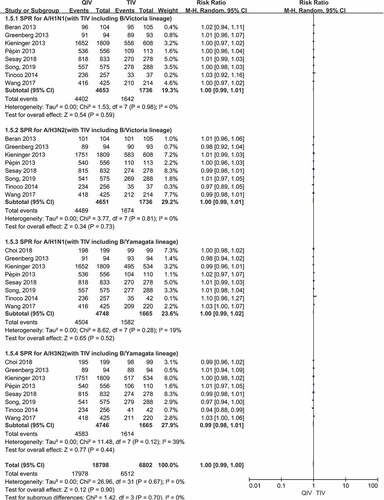
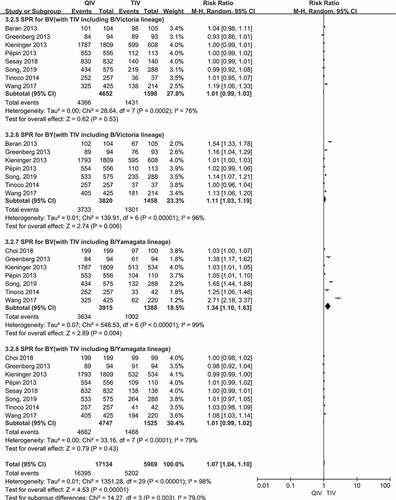
Figure 3. (a,b). Seroconversion rate (SCR) at day 21 post-vaccination, QIV versus TIV in adults between 18 and 64 years old (A strains). (SCR is defined as the percentage of participants with either a pre-vaccination HAI titer <10 and a post-vaccination HAI titer ≥40 or a pre-vaccination HI titer ≥10 and a ≥4-fold increase in HI titer after vaccination)