Figures & data
Figure 1. Most of the threatening infectious disease is targeted at the mucosal sites (around 400 mt2), therefore, the interaction at these sites constitutes the first line of the host defense. Upon host–pathogen interaction, a differential outcome for each other is the results of A + B, the product of this interaction, can be visualized as the outcome of the transcriptional and genomic program for the induction of protective adaptive immune response (HOST).; and for another side as an adjustment and fitness of the genomic program to develop evasion mechanism (PATHOGEN) for the successful establishment, survival, and adaptation into the host
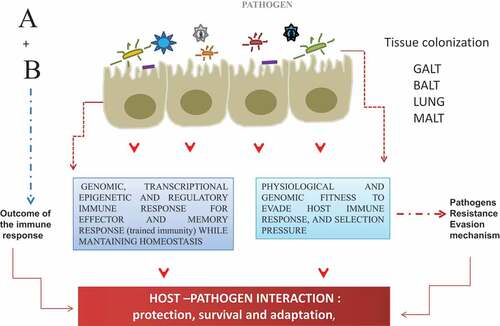
Figure 2. The repertoire of adjuvants compounds, categories, and development since 1920. A released system developed to target the vaccine subunits to the sites of the induction of the innate immune response (AS03, AS04, liposomes virosomes, viral-like particles). TLR agonist, immunostimulants (CpGODN, ISCOMS; Poly (I: C), MPL Flagellin; QS21, Imiquimod, Resiquimod). Of relevance is the availability and the potential of molecular mucosal adjuvants, -recombinant bacterial toxin, type I IFNs-that deserve further exploration and deep evaluation in phase I through phase III against intracellular pathogens
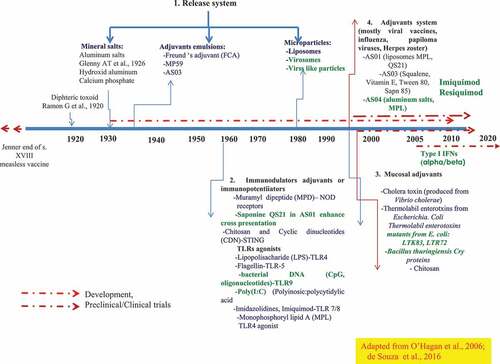
Figure 3. How adjuvant compounds in vaccine formulations initiate, boost and trigger the innate and adaptative immune response. The induction of the immune response occurs through the interaction of the mimic pathogen-associated molecular patterns (PAMPs) and the PRRS (e.g. TLRs, NLRs receptors) on antigen-presenting cells (APCs) (dendritic cells) that trigger an innate immune response leading to activation and maturation of APCs and initiation of downstream MyD88 signalization transduced to the nucleus, leading to pro-inflammatory response necessary to mount a specific durable and effective immune response by T CD4+ and T CD8+ lymphocytes (a). The mechanism of action of the adjuvants comprises a program of four signals which ended with the induction of the effector and memory cellular immune responses and thereby specific T and B cell responses. From the repertoire of adjuvants (TLR agonists, CpG-ODN, Poly(I: C, bacterial toxins), vehicles/carriers (ISCOMS, virosomes, liposomes), adjuvant system (AS03, AS04) as components of modern vaccines, which usually lack some of the components of the whole live microorganism, initiate the innate immune response by acting as PAMPs and thereby, participate by enhancing the interaction between the vaccine ((Ag) and the antigen-presenting cells (macrophages, dendritic cells, epithelial cells), by enhancing uptake, presentation, costimulation and activation, n of quality and magnitude of the activation of CD4 + T cells and CD8 + T cells and B cell differentiation to plasmacytoid B cells antibody producers, cytokines (TGF-β) and T cell homing molecules at MAL (mucosal-associated tissues) (b)
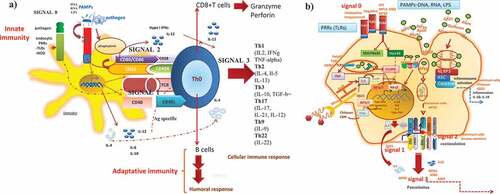
Table 1. Approved TLR agonist as adjuvants
Table 2. Adjuvants in clinical phase studies of human infectious disease vaccines
Table 3. From old to new adjuvants compounds in preclinical testing (animal studies) for human vaccines
