Figures & data
Table 1. Professional characteristics and practices among a sample of ACOG fellows, N = 321
Table 2. Vaccine information accessibility and package insert impressions among a sample of ACOG fellows, N = 321
Figure 1. Initial perception of recommending use in pregnant women among ob-gyns in the US: vaccine with Pregnancy Categories labeling vs. vaccine with Pregnancy and Lactation Labeling Rule (N = 321)
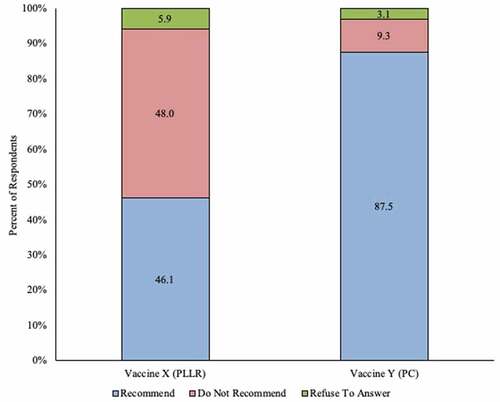
Figure 2. Initial perception of safety of use in pregnant women among ob-gyns in the US: Vaccine with Pregnancy Categories labeling vs. vaccine with Pregnancy and Lactation Labeling Rule (N = 321)
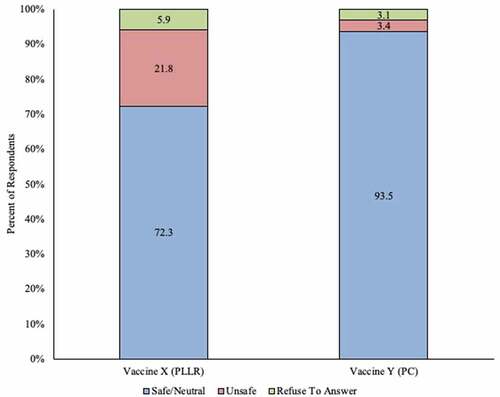
Table 3. Association of professional characteristics and practices with reading vaccine package inserts among a sample of ACOG fellows, N = 304*
Figure 3. Overall opinion on and awareness of utility of Pregnancy Categories and Pregnancy and Lactation Labeling Rule among ob-gyns in the US
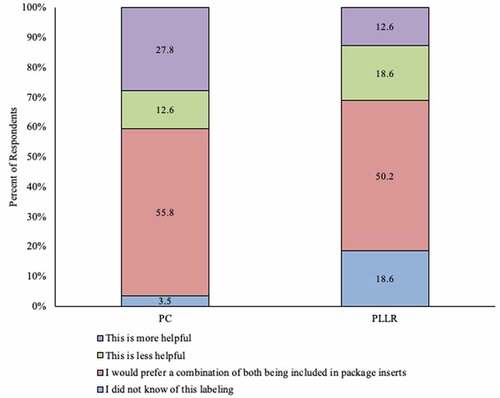
Table 4. Themes identified from open-ended question ‘What are your thoughts and comments about package inserts’ safety statements under “pregnancy and lactation” for vaccines that are recommended for use in pregnancy?’.
