Figures & data
Figure 1. The mechanism of nucleic acid-mediated immune stimulation. The presence of naked mRNA/DNA from any source e.g. infectious organisms, diet or cellular DNA fragments, are sensed by a protein complex. Successive downstream signaling induces type-I interferon, which further stimulates the production of interferon stimulation genes (IGS). Abnormal expression of IGS might link to different immunological disorders (IDs). While second-generation mRNA vaccinations should be safer and may not strongly associated with this mechanism, long-term exposure to any nucleic acid in the form of vaccination may activate the nucleic acid-mediated immune sensing pathway, raising the risk of IDs
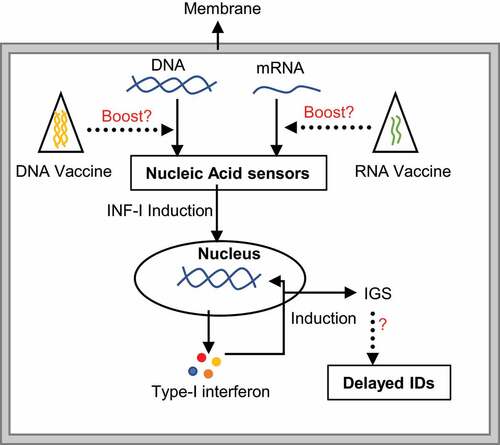
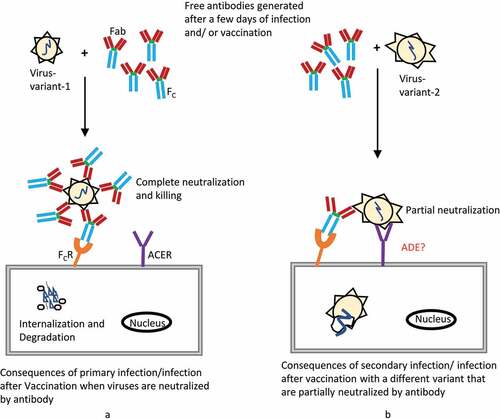
Figure 2. Schematic representation of antibody action and antibody dependent enhancement (ADE) to viral infection. (a) Antibodies generated after a few days of viral infection or successful vaccination, which in turn neutralize the viruses, enabling protection from further infection. (b) Secondary infection or infection after vaccination with a different viral strain causes failure of complete neutralization by the previously existing antibody. However, due to sharing some antigen similarities, partial neutralization may accelerate viral internalization by antibody-binding Fc receptors. Although nucleic-acid-based COVID-19 vaccines have been successfully approved and have shown no ADE complications in trials, including non-human primate animal studies, vaccinated individuals should be monitored across time