Figures & data
Table 1. Baseline characteristics
Figure 1. Changes in geometric mean titer from baseline among adults in Russia administered 2 doses of varicella vaccine 6 weeks apart. A titer of ≥5 gpELISA units/mL 6 weeks after vaccination is considered an approximate correlate of protection for individual vaccinees.
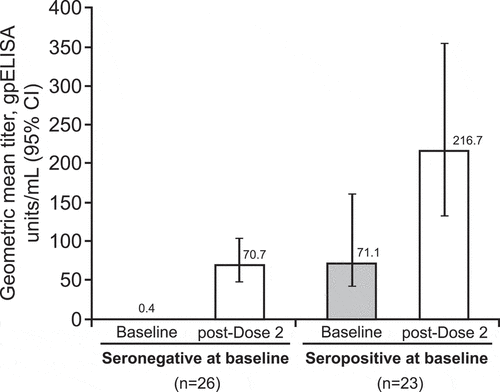
Table 2. Injection-site adverse events
Table 3. Systemic adverse events
