Figures & data
Figure 1. The management of active RRMS is based on the disease activity and prognostic factors. (A) Following the diagnosis of RRMS, treatment with disease modifying therapy (DMT) with moderate efficacy and good safety profile is initiated. In the event of occurrence of disease activity (in the form of relapses or new T2 or Gd+ lesions), a DMT with higher efficacy is usually initiated. This approach in sequencing of different DMTs depending on their efficacy/safety profile is called the escalation approach. (B) If the patient has highly active RRMS or poor prognostic factors, treatment with high-efficacy DMTs is started from the diagnosis of RRMS. Monoclonal antibodies which have the potential to act as an immune reconstitution therapy (alemtuzumab and ocrelizumab) are frequently used in this situation.
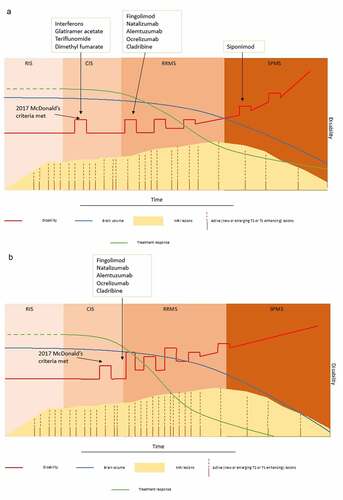
Table 1. Main outcomes of core clinical trials with alemtuzumab in RRMS and ocrelizumab in RRMS and PPMS
Table 2. Main outcomes of extension studies with alemtuzumab in RRMS
Table 3. Main outcomes from real-world studies with alemtuzumab in RRMS and ocrelizumab in RRMS and PPMS
Table 4. Adverse events associated with alemtuzumab and ocrelizumab
