Figures & data
Figure 1. Protection from both ends of the antibody.
Schematic representation of how both ends of the antibody can protect host cells from viral infection. On one end (above the dotted lines), antibodies can block virus entry into the cells by Fab-mediated neutralization. On the other end (below the dotted lines), the antibody Fc domain can recruit different Fc receptors (in light brown) and induce a variety of immune responses (in gray) by innate immune cells (names in blue) and complement.
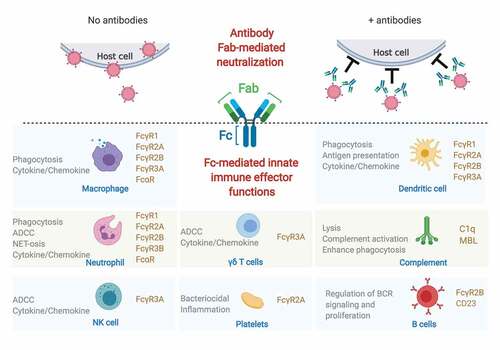
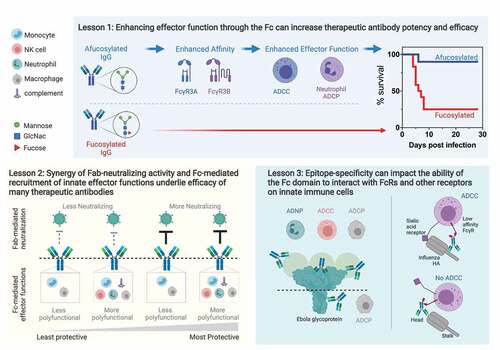
Figure 2. Lessons from monoclonal antibody therapeutics against viruses.
Lesson 1. Enhancing effector function through the Fc can increase therapeutic antibody potency and efficacy. Afucosylated antibodies (above dashed line), indicated by the lack of fucose on the Fc glycan (red triangle), demonstrate increased affinity for FcγR3A and FcγR3B, which in turn confers elevated levels of effector functions such as ADCC and ADCP compared to fucosylated antibodies (below dashed line). In animal models, afucosylated antibodies (blue line) can show increased protective efficacy compared with fucosylated antibodies (red line).Lesson 2. Synergy of Fab-neutralizing activity and Fc-mediated recruitment of innate effector functions underlie efficacy of many therapeutic antibodies. Left to right: antibodies are ordered by protection in animal models, where antibodies that have less neutralizing/no neutralizing activity and low levels of effector functions are less protective than antibodies with less neutralizing/no neutralizing activity but high levels of effector functions. In the context of neutralizing antibodies, increased protective efficacy may be achieved by recruiting more Fc functions and neutralizing antibodies can maximize the efficacy through polyfunctional Fc profiles.Lesson 3. Epitope-specificity can impact the ability of the Fc domain to interact with FcRs and other receptors on innate immune cells. In Ebola, antibodies that bind to the top tier of the glycoprotein and/or the secreted glycoprotein (sGP) induce multiple effector functions, such as high levels of ADCC and ADCP; whereas those targeting the base of the glycoprotein were limited to phagocytic functions. In influenza, induction of ADCC requires interaction with the FcγR through the antibody Fc and a sialic acid receptor on the effector cell that binds to the head domain of hemagglutinin (HA). Antibodies that bind to the stalk domain of HA can induce ADCC activity as the sialic acid receptor is able to bind to the head domain of HA, but the interaction with the sialic acid receptor is blocked when an antibody is bound to the head domain, thus preventing ADCC.