Figures & data
Figure 2. Trial profile and demographic characteristics of vaccinated study participants. PPI, per-protocol population for immunogenicity.
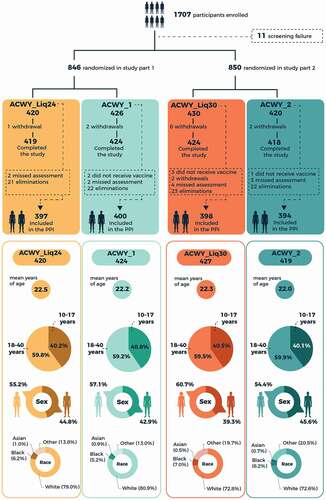
Table 1. Adjusted hSBA geometric mean titers (GMTs) 1-month post-vaccination and between-group ratios for groups that received MenACWY-CRM liquid vaccine aged for 24 or 30 months (ACWY_Liq24 and ACWY_Liq30) versus groups that received licensed MenACWY-CRM vaccine (ACWY_1 and ACWY_2) (per-protocol population for immunogenicity)
Figure 3. Percentages of participants (with 95% confidence intervals) with (a,b) human serum bactericidal assay (hSBA) titer ≥8 at 1-month post-vaccination and (c,d) four-fold increase in hSBA titer from baseline to 1-month post-vaccination against serogroups A, C, W, and Y (per-protocol population for immunogenicity).
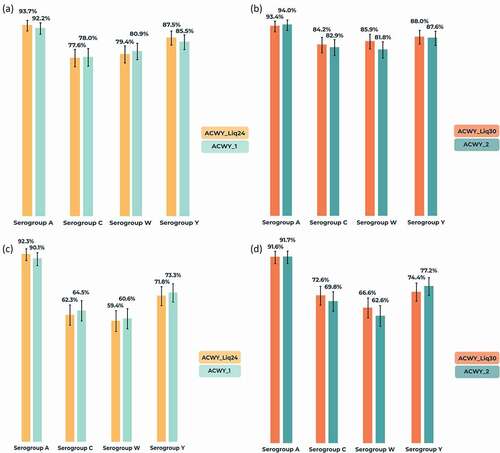
Figure 4. Percentages of participants reporting solicited local and systemic adverse events within 7 days of vaccination (solicited safety population).
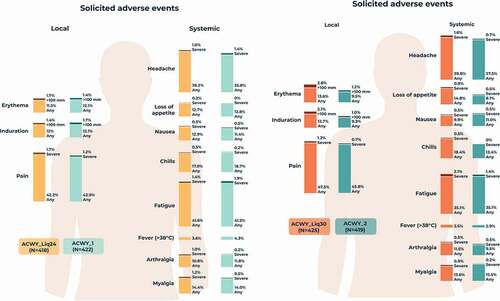
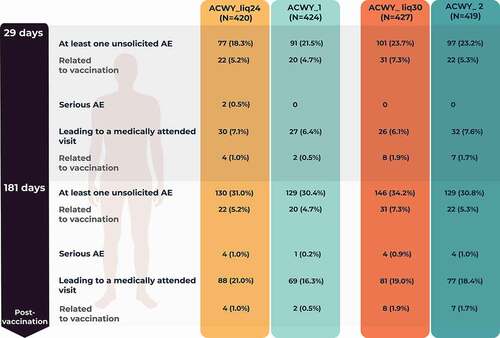
Figure 5. Number (percentage) of participants reporting unsolicited adverse events (AEs) during the 1-month and 6-month post-vaccination periods (unsolicited safety population).