Figures & data
Table 1. Quebec vaccination schedule up to 24 months of age as applicable for children in this study
Figure 1. Vaccination coverage of children who turned the milestone ages each month from January to November in 2019 and 2020. VC assessed by 3 months old (Panel A) and 5 months old (Panel B) for the first and second doses of DTaP and rotavirus vaccines scheduled at 2 and 4 months old. *P-value < .05 for the comparison in VC between 2019 and 2020 using a chi-square test.
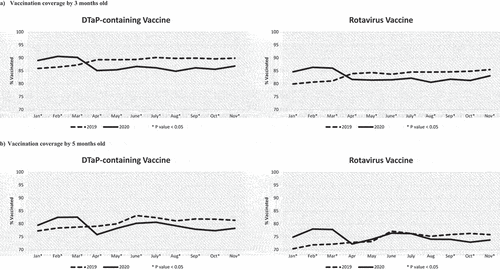
Figure 2. Vaccination coverage of children who turned the milestone ages each month from January to November in 2019 and 2020. VC assessed by 13 months old (Panel A) for the first dose of the measles-containing vaccine and for the third dose of the pneumococcal vaccine scheduled at 12 months old, and by 19 months old (Panel B) for the DTaP vaccine and the measles-containing vaccine (second doses) scheduled at 18 months old. *P-value < .05 for the comparison in VC between 2019 and 2020 using a chi-square test.
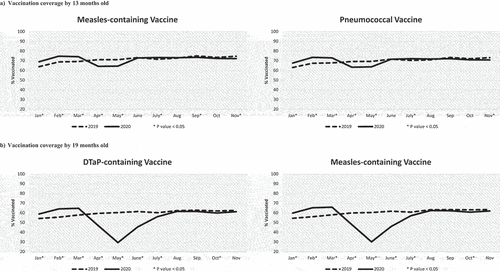
Figure 3. Cumulative proportion of children vaccinated for the first and second dose of DTaP-containing vaccine among children turning 2 months old (Panel A) and 4 months old (Panel B) in March 2019 and 2020 (left) and in April 2019 and 2020 (right). Bars represent 95% Confidence intervals calculated with the exact method.
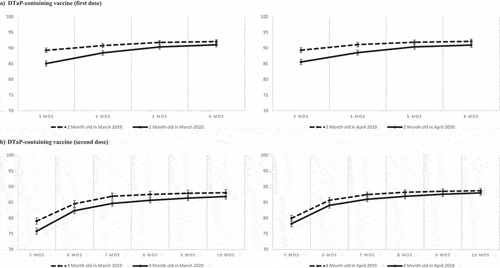
Figure 4. Cumulative proportion of children vaccinated for the first and second dose of measles-containing vaccine among children turning 12 months old (Panel A) and 18 months old (Panel B) in March 2019 and 2020 (left) and in April 2019 and 2020 (right). Bars represent 95% Confidence intervals calculated with the exact method.
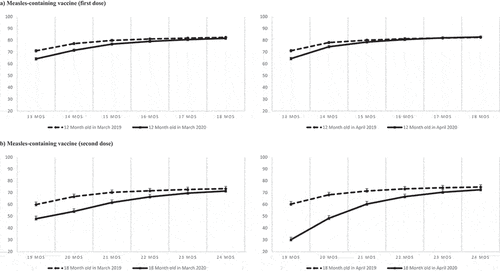
Table 2. Doses of measles-containing vaccines (MMR or MMRV) administered before 24 months of age in 2020 compared with 2019, by month from January to November
