Figures & data
Figure 1. The adaptive immune response to ID BCG vaccination. ID BCG vaccination can arouse a strong adaptive immune response in human body, but these immune responses are not enough to resist Mtb infection for the long term. There are three main reasons: (a). Cellular immunity plays a crucial role in fighting against Mtb infection. Although the T helper 1 (Th1) immune response induced by ID BCG vaccination is relatively robust, it would be inhibited by the Th2 and Treg immune response. In addition, the immune response of Th17 and CD8+ induced by ID BCG vaccination is weak. (b). Recently more and more evidences indicate that humoral immune responses play important roles in protection against Mtb, but the level of antibodies induced by ID BCG immunization is very low, or even almost undetectable. (c). For the memory immune responses, although ID BCG vaccination can induce a large number of effector memory T (TEM) cells, the number of central memory T (TCM) cells and resident memory T (TRM) cells account for small population. Such a composition of memory cells would result in vaccine-induced protection not being sustained for long and make it difficult to respond quickly to the presence of pathogens. The red“ – ” represents a weak immune response, the red“-” represents an adverse immune response, and the red “↓” represents a decrease in the intensity of the immune response.
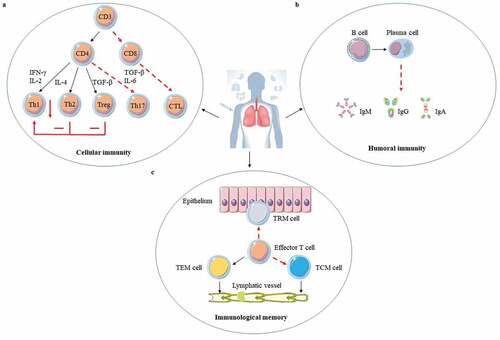
Figure 2. The immune mechanisms of BCG delivered by mucosal and intravenous vaccination. (a). BCG is first taken up by M cells of mucosal epithelium and transported to mucosa-associated lymphoid tissue (MALT). After BCG is processed by dendritic cells, effector T and B lymphocytes are generated, and then differentiated into memory cells. The effector T and B lymphocytes play their protective functions in the effective sites after lymphatic circulation and blood circulation. Except for that tissue-resident memory T (TRM) cells remain constrained within local tissue, central memory T (TCM) cells and effector memory T (TEM) cells migrate to the corresponding lymphatic organs or non-lymphoid tissues. When the body is attacked by Mtb, TRM cells respond quickly, and then the circulating memory cells perform their effector functions. At the same time, memory B cells also rapidly differentiate and secrete IgA (sIgA). (b). Darrah groupsCitation16 showed IV BCG made that 9 out of 10 macaques were highly protected and even 6 showed no signs of infection. The possible protective mechanism of IV BCG vaccination: increased markedly antigen-responsive T cells, higher significantly antibody response, and well-trained immunity. Red shows the presence of bacteria and pulmonary tuberculosis disease, and Orange indicates reduced bacterial burdens and disease, whereas brown remarks no detectable infection.
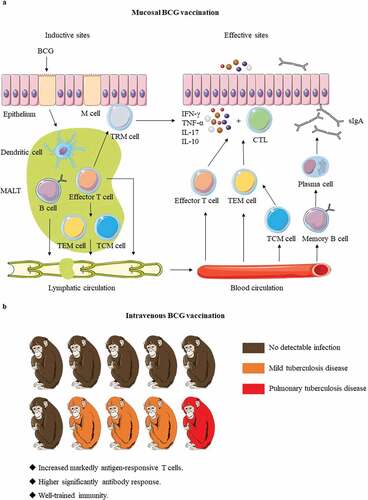
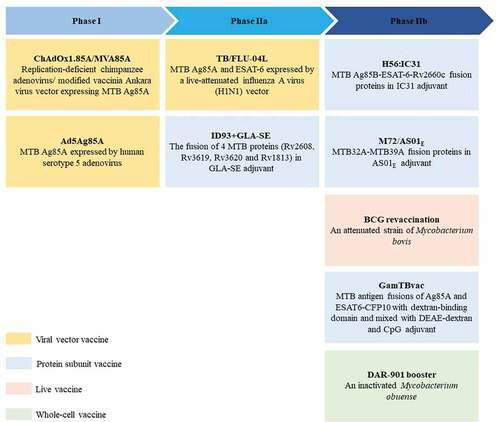
Figure 3. BCG booster vaccine candidates in clinical development. There are currently 9 BCG booster candidates in clinical development, including viral vector vaccines, protein subunit vaccines, live attenuated vaccines, and whole cell vaccines 69–77. The stage of clinical development of vaccine candidates is inferred from data available at ClinicalTrials.gov. Abbreviation: TLR = toll-like receptor.