Figures & data
Figure 1. Flowchart of the selection of patients for comparison of local adverse events following immunization between Japanese encephalitis vaccines.
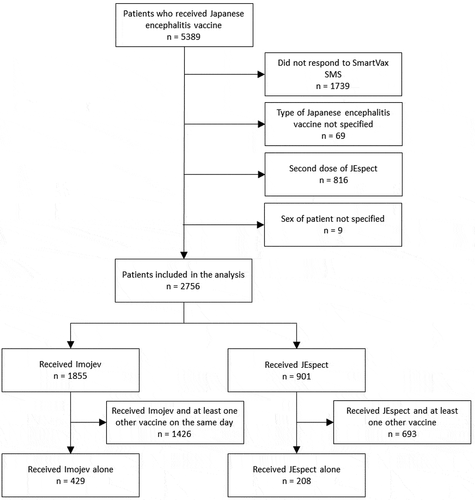
Table 1. Characteristics of patients and adverse events following immunization by type of Japanese encephalitis vaccine received
Figure 2. Percentage of patients who reported overall, systemic, and local adverse events following immunization (AEFIs) by the type of Japanese encephalitis vaccine received.
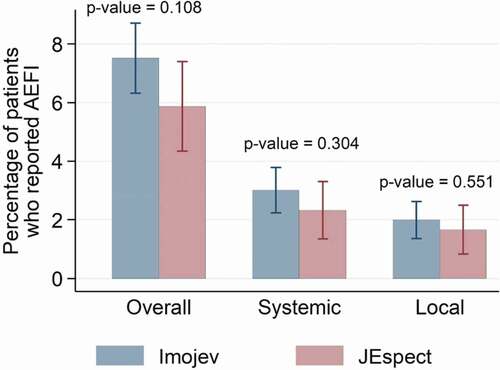
Table 2. Univariate and multivariable logistic regression models for predictors of overall, local, and systemic adverse events following Japanese encephalitis immunization