Figures & data
Table 1. Age distribution of the cases
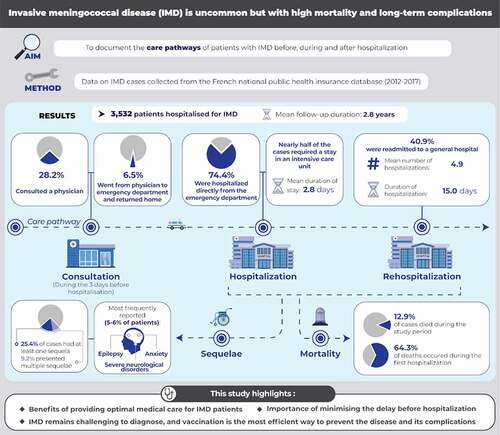
Figure 1. Patient trajectory for management of invasive meningococcal disease. For one patient, discharge destination was unknown.
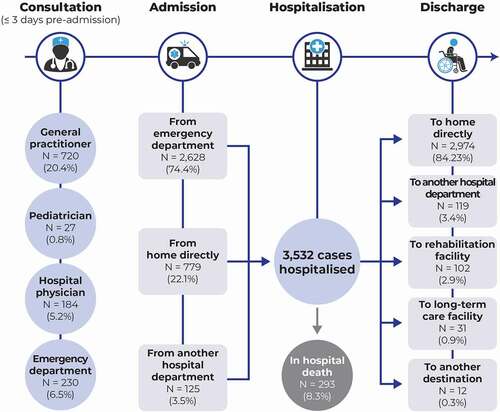
Table 2. Characteristics of index hospitalization stay, by age group
Table 3. Hospital stay duration and in-hospital mortality in children, adolescents, and young adults during the six-year study period
Figure 2. Mortality risk as a function of age in survivors. Hazard ratios for mortality (excluding death during the index hospitalization) in cases compared to controls are presented with their 95% confidence interval.
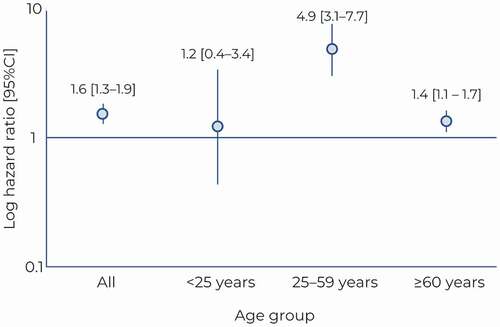
Table 4. Hospitalizations in cases and controls during the follow-up period
Table 5. Sequelae of interest
Figure 3. Sequelae of interest. Data are presented as a Forest plot illustrating the risk ratios for the occurrence of each type of sequela between cases and controls, with their 95% confidence intervals (95% CI).
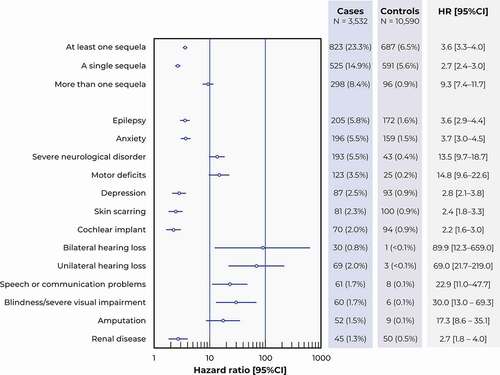
Table 6. Frequency of sequelae as a function of age class
Table 7. Association between number of sequelae and proportion of patients hospitalized or death
Supplemental Material
Download MS Word (18.9 KB)Data availability statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies that evaluate medicines upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an inquiry via the website.