Figures & data
Figure 1. A flowchart of the screening process for randomized controlled trials (RCT) articles of the COVID-19 vaccine.
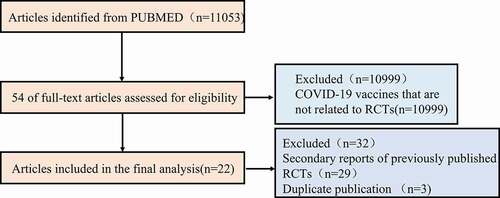
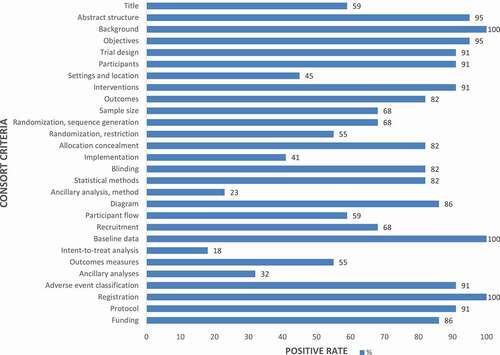
Table 1. Overall quality of reporting: rating using items based on the 2010 CONSORT statement (n = 22)
Table 2. The basic characteristics of clinical experiments
Table 3. Trial characteristics
Table 4. Publication characteristics associated with 2010 overall reporting quality
Supplemental Tables
Download Zip (47.5 KB)Data availability statement
Data are available upon reasonable request.