Figures & data
Figure 1. Schematic representation of the presence of immunoglobulins in infants due to maternal vaccination. (a) Maternal IgG is selectively transported across the placenta by the neonatal Fc receptor (FcRN). (b) Maternal vaccines augment or induce maternal antibody levels to protect the infant from infectious disease in the first few months of life.
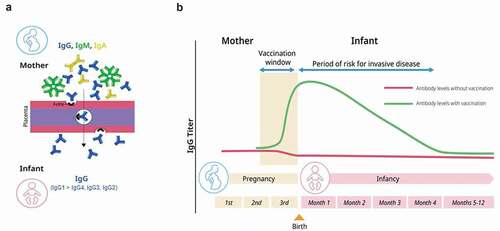
Figure 2. Global prevalence of GBS serotypes causing neonatal disease (2004–2013). (a) Distribution of serotypes by region. (b) Overall global distribution. Adapted from Buurman et al.Citation46
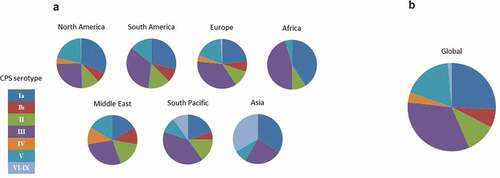
Figure 3. Serotype-specific IgG geometric mean fold rise (GMFR) from baseline at 1, 3 and 6 months following GBS6 vaccination for 120 μg (20 μg CPS/serotype/dose) dose level formulated without aluminum phosphate.
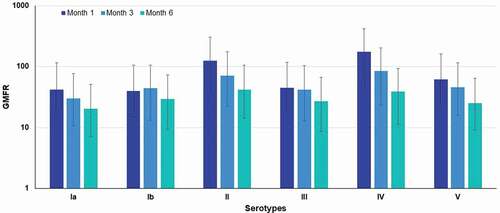
Table 1. Summary of prior seroepidemiological studies for GBS
Table 2. Assay formats and reference standards that have been used to quantify anti-GBS capsule polysaccharide IgG antibodies in published seroepidemiological studies
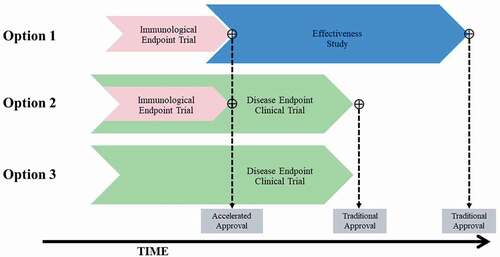
Figure 4. Overview of potential approaches to licensure of a maternal GBS6 vaccine. Effectiveness study refers to a clinical endpoint trial that is conducted under real-world settings after vaccine licensure. Disease endpoint clinical trial refers to an efficacy trial with GBS disease as the primary study endpoint. Accelerated approval is not applicable to Option 3.