Figures & data
Table 1. Participant baseline characteristics
Figure 2. Percentage of participants with solicited adverse events after each vaccination, by severity.
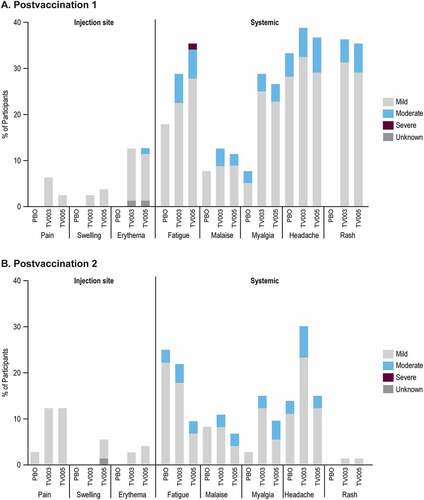
Figure 3. Vaccine viremia after each vaccination, stratified by baseline flavivirus serostatus.
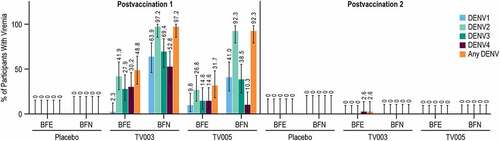
Figure 4. Longitudinal viral reduction neutralization test (Vrnt60) seropositivity frequency, stratified by baseline flavivirus serostatus.
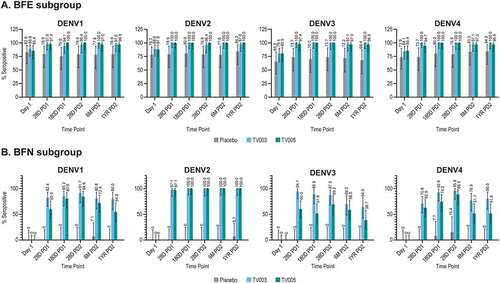
Figure 5. Valency of viral reduction neutralization test (Vrnt60) seropositivity, stratified by baseline flavivirus serostatus.
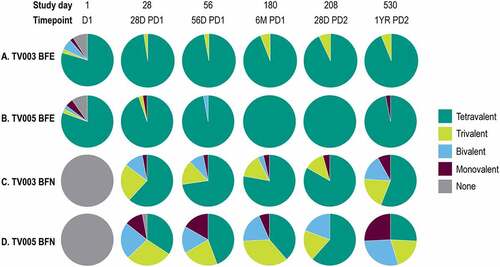
Figure 6. Longitudinal geometric mean titers stratified by baseline flavivirus status.
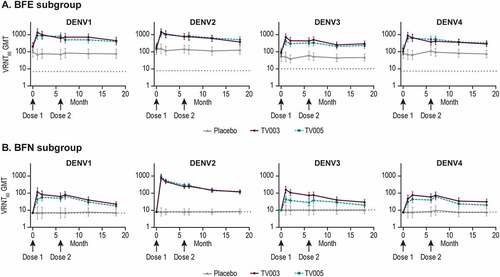
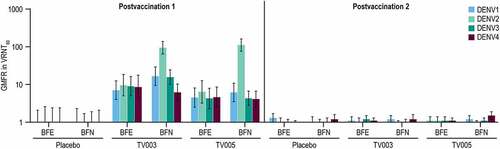
Figure 7. Geometric mean fold rise in VRNT60 stratified by baseline flavivirus serostatus.