Figures & data
Figure 1. Participants’ flow. Eighty-eight participants in the study group were enrolled. Forty-four participants were assigned to CoronaVac/azd1222 group and 44 to AZD1222/CoronaVac group. Twenty participants lost to follow-up, 10 in CoronaVac/azd1222 group and 10 in AZD1222/CoronaVac group. a comparison group was collected from health care workers who completed 2 doses of CoronaVac and available immunogenicity data. 136 health care workers with age and sex matching were chosen for analysis.
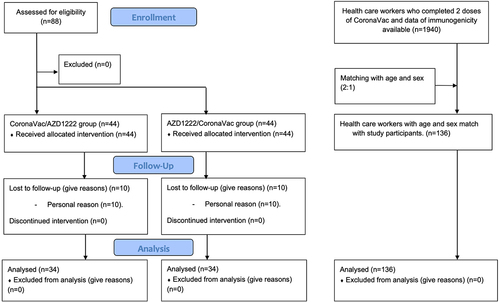
Table 1. Demographic and clinical characteristic at baseline of all participant in each cohort
Figure 2. Anti-Receptor binding domain (RBD) total antibodies 1 month after the second dose of COVID-19 vaccine. AZ: AZD1222 vaccine. SV: CoronaVac vaccine. SV/SV: prime with CoronaVac, boost with CoronaVac. AZ/SV: prime with AZD1222, boost with CoronaVac. SV/AZ: prime with CoronaVac, boost with AZD1222.
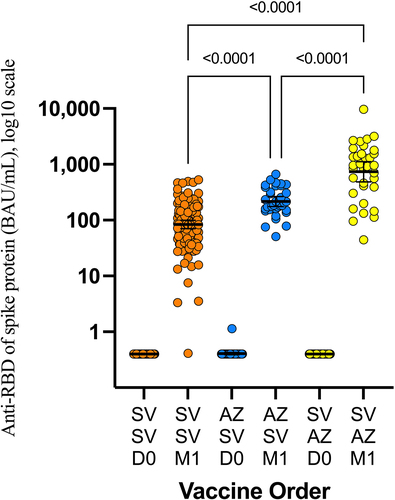
Table 2. Geometric mean ratio of anti-RBD of the spike protein of SARS-CoV-2 at 4-week after the 2nd dose of heterologous prime-boost approach
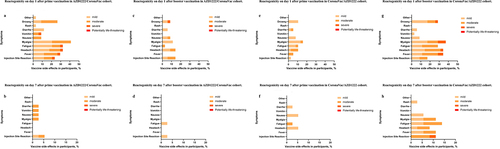
Figure 3. Reactogenicity within 7 days after vaccination. (a) Reactogenicity on day 1 after prime vaccination in AZD1222/CoronaVac cohort. (b) Reactogenicity on day 7 after prime vaccination in AZD1222/CoronaVac cohort. (c) Reactogenicity on day 1 after booster vaccination in AZD1222/CoronaVac cohort. (d) Reactogenicity on day 7 after booster vaccination in AZD1222/CoronaVac cohort. (e) Reactogenicity on day 1 after prime vaccination in CoronaVac/azd1222 cohort. (f) Reactogenicity on day 7 after prime vaccination in CoronaVac/azd1222 cohort. (g) Reactogenicity on day 1 after booster vaccination in CoronaVac/azd1222 cohort. (h) Reactogenicity on day 7 after booster vaccination in CoronaVac/azd1222 Cohort.