Figures & data
Table 1. Summary of the age distribution of 4vhpv vaccine recipients, October 2009 to December 2016.
Table 2. Summary of the Incidence Rates (IR) and Relative Rates (RR) for health outcomes significantly increased among 4vhpv vaccine recipients (N = 114,035) (All doses combined).
Table 3. Summary of the Incidence Rates (IR) and Relative Rates (RR) for health outcomes significantly increased among 4vhpv vaccine recipients (N = 114,035) (Dose 1 only).
Table 4. Rate of Venous Thromboembolism (VTE) Outcomes (per 10,000 Person-Years) among 4vhpv vaccine recipients in Risk Period versus Post-Vaccination Self-Control Period by Follow-up Window: All Doses Combined.
Table 5. Rates of Emergent Outpatient, Emergency Room Visit, and Hospitalization Day 0 Outcomes among 4vhpv vaccine recipients and Concurrent Controls with Td/Tdap, Meningococcal, or Influenza Vaccine.
Figure A1. 4vhpv doses by month* (2009–2016).
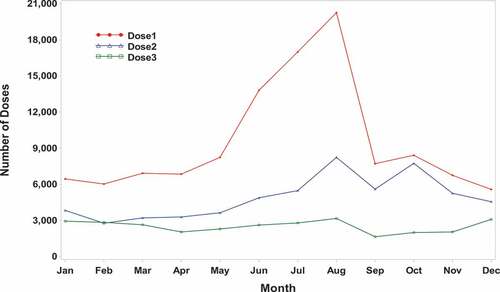
Table A1. Summary of the Incidence Rates (IR) and Relative Rates (RR) that are significantly decreased among regimen initiators (N = 114,035)a,b in a risk period compared to a post-vaccination self-comparison period for potential combined ER/Hospital general safety outcomes by analysis categoryc.
Table A2. Summary of the Incidence Rates (IR) and Relative Rates (RR) that are significantly decreased among regimen initiators (N = 114,035)a,b in a risk period compared to a post-vaccination self-comparison period for combined potential hospital only general safety outcomes by analysis categoryc.