Figures & data
Figure 1. Study plan.
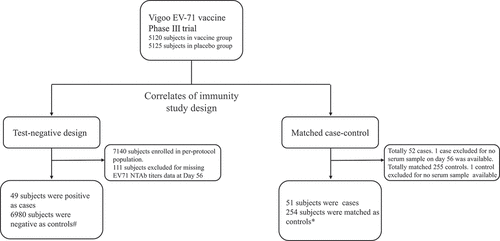
Table 1. Characteristics of cases and controls in test-negative design and matched case-control design.
Figure 2. Reverse cumulative curves for enterovirus 71 (EV71) neutralizing antibody titers about TND and MCC.
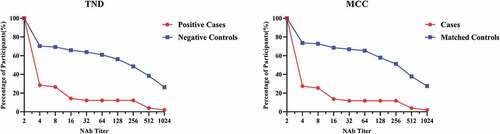
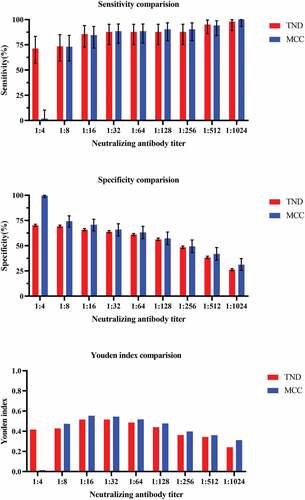
Table 2. Sensitivity and specificity of different cutoff neutralizing antibody titer against EV71associated disease by test-negative design and matched case-control design.