Figures & data
Table 1. Baseline and demographic characteristics of the participants.
Figure 1. Trial profile. The dose-escalation phase 1 study was carried out in three stages. Seven days after the first dose of vaccination in each stage, total adverse reactions and events that occurred during the first week were collected and analyzed. If no vaccine-related serious adverse events occurred within the first week, the next stage of study was started. All the participants received three doses of the allocated vaccine according to the protocol.
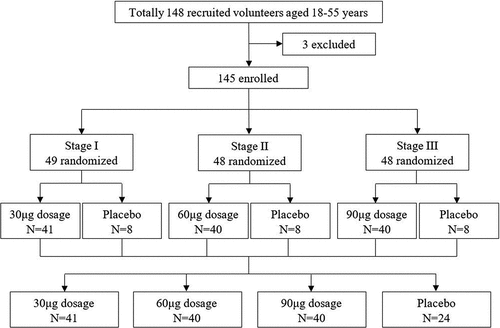
Table 2. The symptoms of adverse events occurred within 30 days after each vaccination.
Table 3. The changes of paired blood routine, serum biochemical, and urine routine indexes before and at 2 days after the first and the third vaccination.
Figure 2. Changes in routine paired blood, serum biochemical, and urine indexes before and 2 days after the first and third vaccinations. The fluctuations were classified into three categories: “maintaining” indicated no grade change observed; “worsening” indicated a change from normal to abnormal or an increase in grade; and “improving” indicated a change from abnormal to normal or a decrease in grade. a) Six routine blood indexes were measured: white blood cell count (WBC), lymphocytes (LY), absolute neutrophil count (ANC), eosinophils (EOS), platelets (PLT), and hemoglobin (HGB). b) Four serum biochemical indexes were measured: total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and glucose (GLU). c) Three routine urine indexes were measured: urinary protein (PRO), urinary glucose (UGLU) and urine occult blood (BLD).
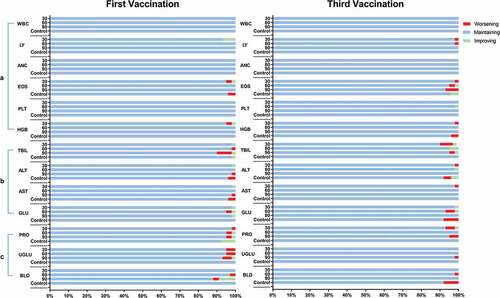
Figure 3. HPV-6/11 antibody levels at one month post three doses of vaccine (month 7) in the per-protocol set. Antibody level of each participant is shown (A: HPV-6 IgG; B: HPV-11 IgG; C: HPV-6 nAb; D: HPV-11 nAb). The black lines indicate the GMC/GMT and 95%CI. *: Significant difference between the two dose groups; **: Significant differences between this group and all the other dose groups. nAb: neutralizing antibody. ID50: the highest dilution which blocked 50% of green fluorescent protein (GFP) expression (50% neutralization).
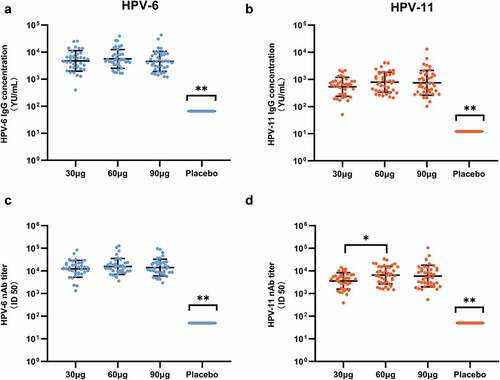
Table 4. Antibody responses at month 7 in participants who received three vaccine doses and were seronegative for antibody against the relevant types of HPV at day 0.
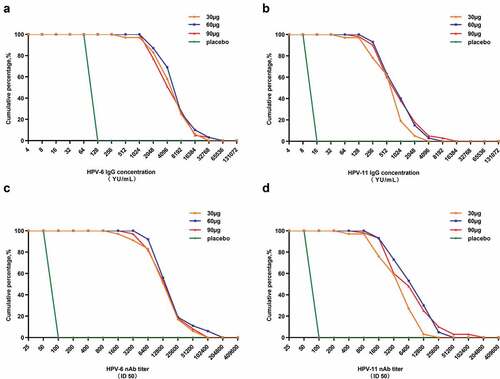
Figure 4. Reverse cumulative distribution curves of the HPV-6/11 antibodies in the per-protocol set for immunogenicity (PPS-I). A: HPV-6 IgG; B: HPV-11 IgG; C: HPV-6 nAb; D: HPV-11 nAb. nAb: neutralizing antibody. ID50: the highest dilution which blocked 50% of green fluorescent protein (GFP) expression (50% neutralization).