Figures & data
Table 1. Efficacy of CoronaVac in clinical trials.
Table 2. Effectiveness of CoronaVac in the real world studies.
Table 3. Safety of CoronaVac in clinical trials and real world surveys.
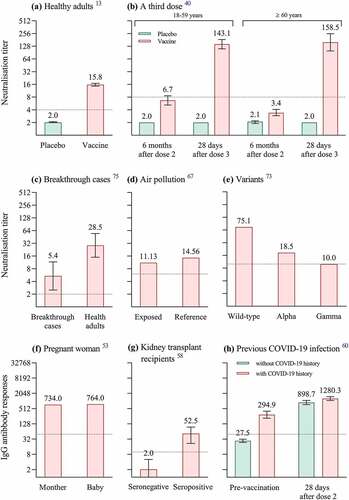
Figure 1. Immunogenicity of CoronaVac in different populations. We summarize immunogenicity of two-dose CoronaVac in healthy population aged 18–59 years (a) and three-dose CoronaVac in healthy population aged ≥18 years (b); immunogenicity of two-dose CoronaVac in breakthrough cases (c), in participants exposed to air pollution (d), against SARS-CoV-2 variants (e), in pregnant woman (f), in kidney transplant recipients (g) and in healthcare workers with previous COVID-19 infection (h). Numbers above the bars in panels a-h are geometric mean titers of neutralizing antibodies against SARS-CoV-2 (a-c, e), median of neutralizing antibody titers (Au/ml) (d), anti-RBD antibody levels (Au/ml) (f), median of anti-SARS-CoV-2 IgG levels (Iu/ml) (g), geometric mean titers IgG antibody of viral spike protein (h). The error bars in panels a-c, h indicate 95% confidence intervals, while those in panel g indicate interquartile ranges. All dotted line denotes the cutoff level for positivity.