Figures & data
Table 1. Overview of qHPV and 9vHPV vaccine clinical trials in India.
Figure 1. Participant disposition for Indian participants in the qHPV vaccine (Study V501–029) (a) and 9vHPV vaccine (Study V503–002) (b) clinical trials.
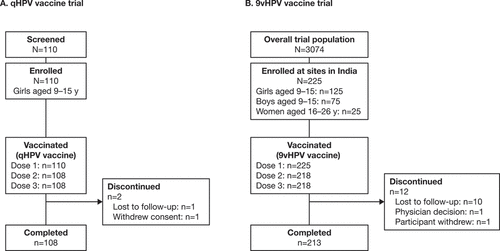
Table 2. Participant baseline characteristics for Study V501–029 and V503–002 (Indian participants).
Table 3. Summary of anti-HPV6/11/16/18 GMTs and seroconversion at Month 7 in Indian girls receiving qHPV vaccine in Study V501–029 and the overall population of young women from Study V501–015 (per-protocol immunogenicity populationa).
Table 4. Summary of anti-HPV6/11/16/18/31/33/45/52/58 GMTs through Month 36 in Indian girls and boys receiving 9vHPV vaccine in Study V503–002 versus young women from the pivotal 9vHPV vaccine efficacy study (Study V503–001) (per-protocol immunogenicity populationa).
Table 5. Summary of anti-HPV6/11/16/18/31/33/45/52/58 GMTs and seroconversion at Month 7 in Indian young women versus the entire cohort of young women in Study V503–002 (per-protocol immunogenicity populationa).
Table 6. Summary of AEs in Indian girls (aged 9–15 years) from the qHPV vaccine study (Study V501–029) (Safety population).
Table 7. Summary of AEs in Indian girls (aged 9–15 years), boys (aged 9–15 years), and young women (aged 16–26 years) from the 9vHPV vaccine study (Study V503–002) (Safety population).
