Figures & data
Figure 1. Schematic illustration of CAR structure. CAR contains an ectodomain possessing antigen recognition domain known as scFv and a short portion connecting it to TMD called hinge region (or spacer). scFv is made from VL and VH of an antibody that is connected to each other by a flexible linker. It also harbors a lipophilic alpha-helical domain spanning the plasma membrane known as TMD. CAR has also an endodomain comprising a CD3ζ that entails three ITAMs responsible for transmitting a primary signal, and CM mediating secondary or costimulatory signals.
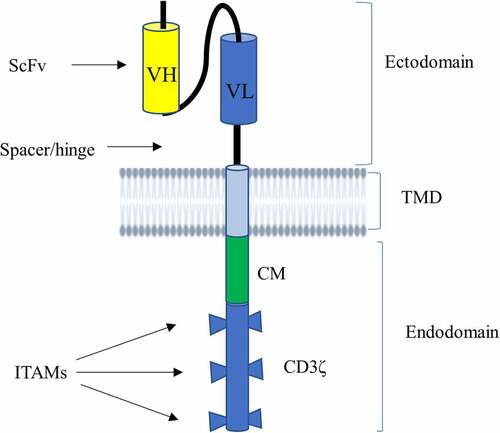
Figure 2. Diagrammatic representation of the structure of CAR from the first generation to the fifth generation. 1G CAR contains only a CD3ζ in its signaling domain with no CM. 2G CAR harbors CD3ζ and one CM, allowing dual signaling pathway.3g CAR, on the other hand, combines CD3ζ and several CMs that deliver multiple signaling. 4G CAR resembles 2G CAR with the incorporation of an additional NFAT-responsive cassette that expresses cytokines playing anti-cancer activities. Thus, 4G CAR has triple signals from primary CD3ζ, CM, and expressed transgenic proteins. 5G CAR is based on 2G CAR by adding membrane receptors IL-2Rβ that provides a binding site for STAT3 and activates the JAK-STAT signaling domain. 5G CAR has also a synergistic activation of triple signals from CD3ζ, CMs, and cytokine-inducing JAK-STAT3/5 pathway.
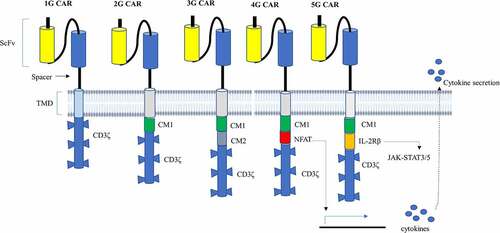
Table 1. A summary table on the generations of car T-cell and their structure, feature, and limitations.
Figure 3. Procedures of CAR T-cell production. (a) T-cell extraction, (b) Genetic engineering (c) CAR T-cell expansion, and (d) Adoptive CAR T-cell transfer.
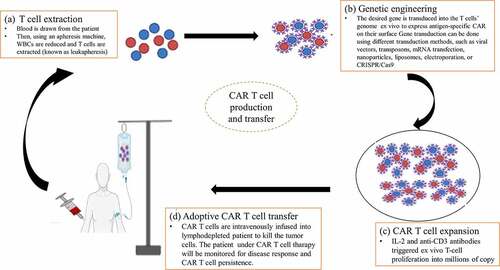
Table 2. Major lymphodepleting regimens for CAR T-cell therapy and their dosages, advantages, and disadvantages.
Figure 4. Mechanism of cancer-killing by CAR T-cell therapy. CAR T-cells detect tumor antigens via its scFv of CAR and become activated and kill cancer cells by using several mechanisms. (a) perforin-granzyme system. Activated CAR T-cells quickly release perforin and GZM from their lytic granules, and then perforin creates membrane pores on cancer cells, allowing GZMs entrance into the cytoplasm of the cancer cells and killing them. (b) the death ligand–death receptors such as the Fas-FasL axis: binding of Fas in the cancer cell membrane to the FasL present on activated CAR T-cells, induce apoptotic cancer cell death. (c) Recruitment of other components of the immune system. Activated CAR-T-cells release soluble factors such as cytokines upon CAR engagement with the target antigen. Secreted cytokines infiltrate tumor cells and cause inflammation eliminating cancer cells, and activate other immune cells such as B-cells and NK cells at the tumor site that aid in the anti-tumor activities.
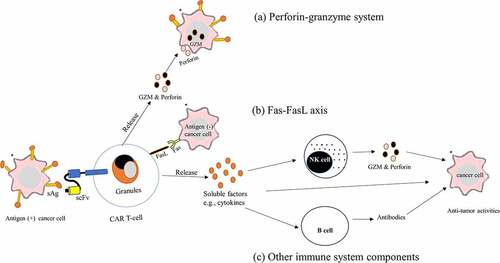
Table 3. Summary of the FDA-approved adoptive CAR T-cell therapies in blood cancers.
Table 4. Some major ongoing clinical trials on CAR T-cell therapy in blood and solid cancers.
