Figures & data
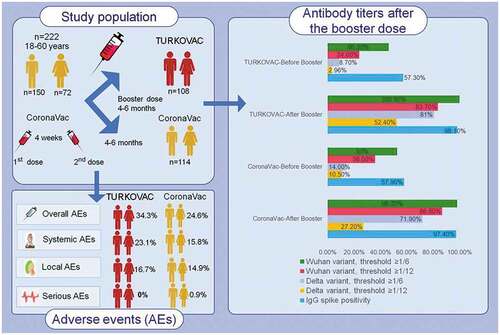
Figure 1. Study flowchart.
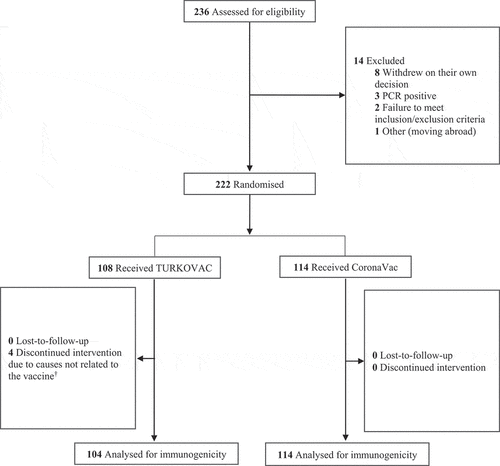
Table 1. Characteristics of the study participants.
Figure 2. Neutralizing antibody positivity against the (a) Wuhan and (b) Delta variants, and (c) immunoglobulin G-Spike (IgG-S) positivity before and after booster doses in the two study arms.
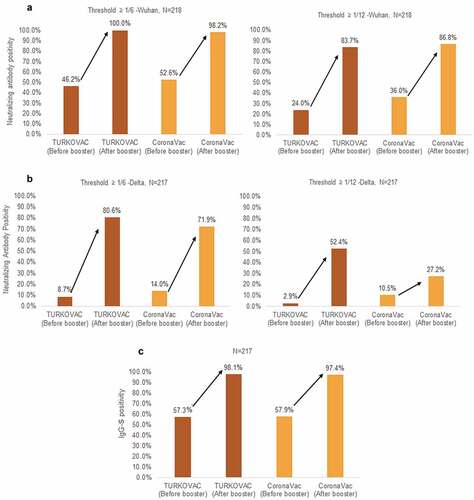
Table 2. Change in spike-specific immunoglobulin G antibody levels before and after the booster doses.
Table 3. Neutralizing antibody geometric mean titer results.
Table 4. Frequency of adverse events in the two study arms.
